
Explore our multimedia assets, recordings, publications, and much more to add to your knowledge of multiple myeloma treatment options and related topics.
Download the MMRF Patient Toolkit—available in English and Spanish—or order a copy through the mail.
Thank you to our sponsors: Amgen, BMS, Genentech, Johnson & Johnson, Pfizer, and Sanofi.

Caregiver Guide
This booklet provides caregivers of multiple myeloma patients with essential guidance to navigate treatment, manage symptoms, and offer support, empowering them to advocate for the best possible care and outcomes.

Newly Diagnosed Multiple Myeloma
This booklet describes some of the first steps you will want to take after receiving a myeloma diagnosis, as well as what you can expect from your treatment team.

Multiple Myeloma Learn Your Labs
This booklet describes these tests and what they mean for you and your health care team.

Multiple Myeloma Treatment Overview
This booklet describes current therapies for myeloma as well as emerging treatment options that are being tested in clinical trials.

Multiple Myeloma Disease Overview
This booklet is designed to help patients with multiple myeloma - and their friends, families, and caregivers - better understand this disease: what myeloma is and how it develops within the body.

Managing My Myeloma Resource Brochure
Find resources that will help you through your journey with multiple myeloma.

Myeloma Precursor Conditions
Patients with multiple myeloma typically have a preceding phase of disease in which there are changes in the bone marrow but no symptoms or organ damage.

Immunotherapy
The use of a patient’s immune system to fight cancer—cancer immunotherapy—is an exciting area of multiple myeloma research.

Autologous Stem Cell Transplantation
Autologous Stem Cell Transplantation (ASCT) remains the standard of care for newly diagnosed, eligible patients.
Note: The MMRF cannot ship to addresses outside the USA.
MMRF Fast Facts in Myeloma are double-sided, one page documents designed to help you
gain a better understanding of multiple myeloma and treatment.
Available in English and Spanish - download now.
Thank you to our sponsors: Amgen, BMS, Genentech, Johnson & Johnson, Pfizer, and Sanofi.

Managing the Financial Impact of Myeloma Care
For multiple myeloma patients, treatments, appointments, supportive care, travel, lodging, and missed work—among many other expenses—can create a big financial burden. This guide provides tips on how to navigate these costs.
Multiple Myeloma Survivorship
With more patients living longer with multiple myeloma, the topic of survivorship is more important than ever. This sheet provides insights on how patients can live and thrive after receiving a myeloma diagnosis.
A Guide to CAR T-Cell Therapy in Myeloma
CAR T-cell therapy is a treatment that uses your body’s own immune system to fight myeloma. This sheet describes the steps involved with CAR T-cell therapy and what treatment-related side effects can develop.

Understanding AL Amyloidosis
Learn about AL amyloidosis, a rare plasma cell disorder that develops in up to 15% of multiple myeloma patients. This sheet describes AL amyloidosis and how it is diagnosed and treated.

Multiple Myeloma: What You Need to Know
An ideal resource for newly diagnosed myeloma patients and their caregivers, this patient information sheet describes what multiple myeloma is, how it affects the body, and what symptoms you may want to discuss with your doctor.

Questions to Ask Your Doctor About Multiple Myeloma
Visits with your care team are the best times to get information that will help you on your myeloma journey. This sheet provides suggestions of questions you can ask about your treatment, available support services, and more.
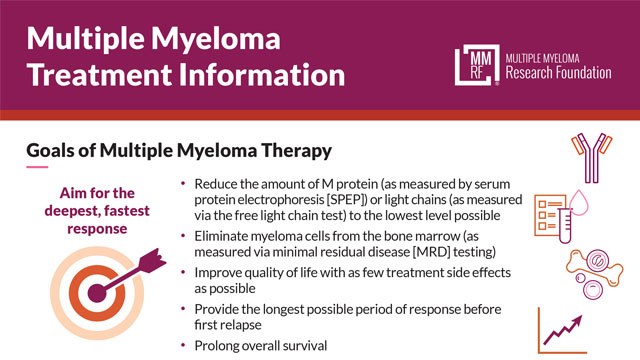
Multiple Myeloma Treatment Information
This sheet describes multiple myeloma treatment goals, how the care team measures a patient’s progress toward reaching them, and the different levels of response to myeloma therapy. Also covered are the treatment approach for newly diagnosed patients and treatment considerations for patients with high-risk myeloma.
Multiple Myeloma Precursor Conditions
What are monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), how do they develop, and what is their relationship to multiple myeloma? These and other important questions are answered in this sheet.
A Patient Guide to Newly Diagnosed Multiple Myeloma
Multiple myeloma is a blood cancer that starts in the bone marrow. This sheet provides information that will help you understand how multiple myeloma is diagnosed, treatment approaches, and available support services.
Multiple Myeloma: Autologous Stem Cell Transplantation
Learn what an autologous stem cell transplant is, what steps are involved, what to expect during recovery, and more.

A Patient Guide to Relapsed/Refractory Multiple Myeloma
Knowing what to expect when multiple myeloma doesn’t respond to treatment (refractory) or returns after treatment (relapse) is important. This sheet describes the options that are available if you have refractory or relapsed myeloma.

A Patient’s Guide to Biomarkers in Multiple Myeloma
Biomarkers play an increasingly important role in helping your doctor manage your myeloma, but what are they and how are they used? This sheet spells it out in easy-to-understand terms.

Multiple Myeloma Risk Assessment: What You Need to Know
What is high-risk multiple myeloma? What tests are used to determine whether a patient is at high risk? This information sheet addresses these questions and more.

Multiple Myeloma Personalized Medicine: What You Need to Know
This sheet describes personalized medicine, an approach to myeloma care that uses detailed information about the patient and their myeloma to tailor treatments that are more likely to be effective and have fewer side effects.

Bispecific Antibodies for Multiple Myeloma: What You Need to Know
This sheet describes bispecific antibodies, an exciting new type of immunotherapy that has shown promise as a multiple myeloma treatment.
Bone Health and Multiple Myeloma: What You Need to Know
Learn how multiple myeloma can affect your bones and what tests and treatments are available to prevent or minimize myeloma-related bone damage.

Multiple Myeloma Patients Getting on The Right Track
The MMRF’s The Right Track program focuses on myeloma patients seeing the right team, having the right tests, and getting the right treatment as quickly as possible after diagnosis. This patient information sheet provides the details.

Clinical Studies in Multiple Myeloma: What You Need to Know
Clinical studies offer hope for many myeloma patients; this sheet describes the different types of clinical studies and answers common patient questions about finding and participating in one
Central to MMRF’s mission is providing educational content no matter where patients and caregivers may be in the multiple myeloma diagnostic and treatment journey. Discover our full range of information options.
Our goal is to share resources and insights on clinical progress and precise treatments for all patients and their caregivers.
To deliver the highest possible level of patient care, the MMRF supports continuous learning for healthcare providers. To keep pace with evolving evidence-based clinical innovations, technologies, and best practices, MMRF provides access to both CME-accredited and non-accredited education and activities.

Challenges and Opportunities for Treatment of Early and Late Relapse in Multiple Myeloma
This interactive activity summarizes key evidence on approved and emerging treatments for early or late relapsed myeloma and provides insights on adverse event (AE) management.
Expires: May 15, 2026

A Step-by-Step Approach to CAR T-Cell Therapy for RRMM: From Bridging to Sequencing and Adverse Event Management
Myeloma experts discuss the latest data and the practical use of CAR T-cell therapy in earlier-line intervention of multiple myeloma.
Expires: Dec 29, 2025

Bridging Gaps in Multiple Myeloma Care: A Community Oncologist’s Guide to Navigating the Evolving Treatment Landscape
Myeloma experts discuss recent updates in the treatment landscape and practical strategies that may be implemented with patients in your community practice.
Expires: Dec 29, 2025

Championing the Care of Relapsed/Refractory Multiple Myeloma: Practical Strategies to Integrate Bispecific Antibodies
Experts in hematology discuss the use of bispecific antibodies for the management of patients with relapsed/refractory multiple myeloma.
Expires: Dec 29, 2025

New Indication for Sarclisa in Myeloma: What HCPs Need to Know – Dr. Jesus Berdeja
Dr. Jesus Berdeja provides expert insights into the FDA's recent approval of a new indication for Sarclisa. Learn how this development impacts treatment protocols and patient care.

Podcast: Bispecific Antibody Horizons: Dosing Strategies and Meeting Updates in Myeloma Care (non-accredited activity)
This is a non-accredited activity.
This episode of Myeloma Matters includes in-depth discussion and analysis of the latest scientific findings and practice-changing advances (from 2024 ASCO and EHA) in managing RRMM.
Expires: August 15, 2025

Podcast: Prevention and Management of Bispecific Antibody–Associated Adverse Events in Multiple Myeloma (non-accredited activity)
This is a non-accredited activity.
This episode of Myeloma Matters features discussions of bispecific antibody therapy, a powerful form of targeted immunotherapy that has produced high response rates and helped patients.
Expires: July 18, 2025

Putting the CAR(T) Before the Horse: Practicalities of T Cell-Activating Therapies in Multiple Myeloma
This webcast features evidence-based presentations along with patient cases designed to address the nuances of T cell—activating therapy use, identification of patients that are the best candidates for these approaches, and options for patients who may not be candidates for this therapy.

What’s My (Next) Line? Treating Relapsed/Refractory Multiple Myeloma When Three Prior Treatment Lines Fail
This activity has been developed to present and discuss current recommended strategies for individualizing treatment plans for patients with RRMM by incorporating the most up-to-date clinical evidence on sequencing small molecule–based therapies and BCMA-targeted therapies that achieve durable remission while minimizing toxicity.

Applying the Latest Clinical Data in Multiple Myeloma Patient Care in the Community Setting (Oncology)

Applying the Latest Clinical Data in Multiple Myeloma Patient Care in the Community Setting (Internal Medicine)