Over three days, the 2025 International Myeloma Society (IMS) Annual Meeting featured dozens of new and updated clinical trial results, illustrating significant advancements in therapy from newly diagnosed (NDMM) to relapsed/refractory multiple myeloma (RRMM).
Topics included real-world CAR T insights, evolving outpatient (OP) and modified bispecific antibody (bsAb) practices, expanding roles for bsAbs in NDMM and smoldering MM (SMM), tailored approaches for high-risk disease, new analyses of quadruplets, growing evidence for cereblon E3 ligase modulatory drugs (CELMoDs) across treatment settings, emerging experimental agents that highlight the importance of clinical trial participation, and a broad set of new biomarkers that refine risk assessment and treatment response prediction.
Real-World Insights Reveal CAR T Benefits and Tradeoffs
Real-world evidence comparing ide-cel and cilta-cel underscored the durable responses and varying toxicity profiles of each CAR T therapy, offering important comparative insights in the absence of head-to-head clinical trials.
Real-world comparison of ide-cel and cilta-cel via CIBMTR data
- Intervention: Ide-cel and cilta-cel are BCMA-directed CAR T-cell therapies approved for RRMM. This was a multicenter comparison using Center for International Blood and Marrow Transplant Research (CIBMTR) data (2021–2023)
- Population: 1,581 pts were included: 986 received ide-cel and 595 cilta-cel. Baseline characteristics were comparable between the cohorts, but the cilta-cel cohort generally had fewer comorbidities, better Eastern Cooperative Oncology Group (ECOG) performance status, lower rates of extramedullary disease (EMD) and penta-exposed disease, and slightly higher response at infusion
- Outcomes: With a median follow-up: 12.0 months for cilta-cel, 12.9 months for ide-cel:
-
- Overall response rate (ORRs) and complete response (CR) rates were higher with cilta-cel: ORR (88.9% vs 75.3%) and CR (36.2% vs 24.4%)
-
- 12-month progression-free survival (PFS) was 72.6% for cilta-cel vs 46.7% for ide-cel and overall survival (OS) was 84.5% vs 73.6%, respectively
-
- Cilta-cel maintained significantly higher odds of ORR (odds ratio [OR] 1.7), CR (OR 1.88), and superior PFS (hazard ratio [HR] 0.47) and OS (HR 0.82)
-
- Cytokine release syndrome (CRS) was of similar frequency overall (79.3% vs 82.1%), but cilta-cel had more grade (G) ≥3 CRS than ide-cel (4.3% vs 2.9%)
-
- Any-grade immune effector cell-associated neurotoxicity syndrome (ICANS) occurred less with cilta-cel (22.3% vs 27.3%), with no differences in severity. Cilta-cel carried additional risk of delayed neurologic toxicities not seen with ide-cel, including parkinsonism (2.7%) and cranial nerve palsies (2.5%)
-
- The 6-month treatment-related mortality (TRM) was higher with cilta-cel (4.0% vs 2.9%). Adjusted analysis confirmed increased TRM risk (HR 1.45)
- Takeaway: Cilta-cel demonstrated deeper and more durable responses, with significantly improved PFS and OS compared with ide-cel. However, the benefits came at the cost of higher TRM, increased rates of severe CRS, and unique delayed neurologic toxicities. Shared decision-making should balance efficacy and safety. Proactive neurotoxicity monitoring for cilta-cel therapy is important to ensure long-term safety and best possible outcomes
Transforming bsAb Care: OP SUD and Other Practices
Multiple real-world studies confirmed that OP or hybrid step-up dosing (SUD) of bsAbs such as teclistamab (tec) and talquetamab (tal) is both safe and practical, especially with prophylactic dexamethasone (dex).
Limited-duration therapy and reduced-frequency regimens, such as less-frequent elranatamab dosing, demonstrated that individualized schedules can maintain efficacy. Notably, a new analysis comparing prophylactic dex + OP SUD vs standard inpatient (IP) observation examined the potential to reduce financial toxicity and resource burden. These results highlight how community practices can deliver advanced therapies while maintaining convenience, safety, and lower costs.
Retrospective cohort study of limited-duration bsAb therapy
- Approach: bsAbs, primarily those targeting BCMA or GPRC5D, were evaluated under a fixed-duration treatment strategy (discontinued treatment without progression)
- Population: 78 RRMM pts (median age: 70, range 26–86) who discontinued bsAbs (72% received BCMA-targeted agents, 19% GPRC5D-targeted agents, and 9% BCMA + GPRC5D-targeted agents) for reasons other than disease progression or death and were alive in remission for at least 3 months post-discontinuation
-
- High-risk cytogenetics: 47%; EMD: 8%
-
- Median duration of bsAb therapy: 7 months; 80% in CR at discontinuation
- Outcomes: At a median 16-month follow-up, relapse-free survival (RFS) at 2 years was 67% (95% confidence interval [CI], 55%–80%). 2-year RFS outcomes varied by prior therapy:
-
- 1–3 prior lines of therapy (LOT), 79%
-
- 4–5 prior LOT, 78%
-
- ≥6 prior LOT, 48%
Factors independently predicting inferior RFS included:
-
- EMD (HR 7.88; P=0.004)
-
- Higher number of LOT (HR 1.79; P<0.001)
-
- Partial remission at discontinuation vs CR (HR 28.3; P=0.012)
The most common causes of treatment discontinuation included infections (44% of pts) and ectodermal toxicities (15%). Post-discontinuation risk:
-
- Cumulative incidence of G≥3 infections was 22% at 1 year, 32% at 2 years, and 38% at 3 years
-
- Risk of first severe infection decreased as the time from discontinuation increased
- Takeaway: Fixed-duration bsAb therapy in RRMM shows that sustained remissions are achievable, particularly in pts with fewer than 6 prior LOT. Randomized controlled trials are needed to determine whether a limited-duration approach is noninferior to treatment until progression
Phase 2 MagnetisMM-3 trial of elranatamab less-frequent dosing in RRMM
- Intervention: Elranatamab administered with priming SUD, transitioning to weekly, biweekly, and then monthly dosing in responders. Prophylactic antimicrobials and immunoglobulin replacement were commonly employed
- Population: 47 heavily pretreated RRMM pts, with a median of 5 prior LOT (range 2–22). Almost all (93.6%) triple-class refractory, and almost half (46.8%) penta-drug refractory
- Efficacy: ORR was 66.0% (95% CI, 50.7–79.1) and ≥CR was 42.6%, with a median time to response (TTR) of 1.08 months and a median time to ≥CR of 4.76 months
-
- Median duration of response (DOR), 40.8 months (95% CI, 24.0–not estimable)
-
- Median PFS, 27.3 months (95% CI, 4.3–not estimable)
-
- Median OS, 43.6 months (95% CI, 14.9–not estimable; not yet mature)
- Safety: All pts experienced an adverse event (AE), and G≥3 AEs were noted in 78.7%. Infections occurred in 70.2% of pts, with 42.6% G3–4. Almost half (51.1%) required immunoglobulin replacement
-
- CRS, 61.7% (G1 34.0%, G2 27.7%, G≥3 0%)
-
- ICANS, 8.5% (all grade 1–2)
- Takeaway: In a heavily pretreated US subgroup of RRMM pts, elranatamab induced deep and durable responses with a less-frequent dosing regimen, including once every 4 wks. Elranatamab is typically administered biweekly (only for pts who have achieved partial response or better) according to the label
Outcomes of OP SUD of tec and tal
- Approach: A retrospective observational study to assess the feasibility and safety of tec and tal treatment initiated with SUD in 3 real-world settings: IP, OP, and hybrid (HY; OP SUD with 48 hours of IP observation)
- Population: 120 RRMM pts treated as follows: OP=23 pts (tec=13, tal=10); IP=54 pts (tec=42, tal=12); and HY=43 pts (tec=29, tal=14). Older pts were more frequent in OP (48% ≥75) than in HY (40%) and IP (31%)
-
- ECOG≥2, OP 9%, IP 19%, HY 17%
-
- High-risk cytogenetics, OP 26%, IP 33%, HY 28%
-
- Heavily pretreated, 70%–81% were penta-drug exposed; median prior LOT ~4–6; prior T cell–redirecting therapy more common in IP (24%) and HY (33%) vs OP (9%)
- Outcomes: All OP pts (tec and tal) completed SUD, and most OP pts (70%) did not require hospitalization during SUD. The median hospitalization for OP pts with G2 CRS was 4 days, and 1 HY pt discontinued due to ICANS; no discontinuations in OP or IP cohorts were noted. CRS emerged within 14 days as follows: OP 61%, IP 50%, HY 63%
-
- Highest grade observed: OP 22% (G2), IP 7% (G2), HY 37% (G2)
-
- Tocilizumab used: OP 13%, IP 17%, HY 56%
-
- No discontinuations due to CRS
ICANS emerged within 14 days as follows: OP 4% (G2), IP 6% (various grades), HY 13% (mostly G1)
-
- Steroids used: OP 0%, IP 4%, HY 14%
-
- No hospitalizations for ICANS; one HY pt discontinued therapy
- Takeaways: OP initiation of tec or tal with SUD is feasible and safe in community oncology practice. Rates and severity of CRS and ICANS were comparable to IP and HY approaches, with manageable toxicity and minimal need for hospitalization
Prophylactic dex with OP SUD of bsAbs vs standard-of-care (SOC) IP observation
- Approach: The cost and feasibility of OP SUD with tec and tal that included prophylactic dex the day after SUD and remote monitoring via Hospital at Home (HaH) with virtual visits, wearable vital sign monitors, and 24/7 nursing access was evaluated and compared to standard 48-hour IP care
- Population: 32 pts received OP care (16 treated with tec and 16 with tal). The comparator group consisted of 24 pts receiving standard IP observation (13 tec, 11 tal)
- Outcomes: CRS incidence was comparable between OP and IP groups (59% vs 54%), with no G3 or 4 CRS observed in the OP group; the IP group had rare G3 and 4 events. Recurrent CRS with subsequent SUDs was less frequent in OP (32% vs 46%). Prophylactic dex did not reduce CRS incidence but may have mitigated severity and reduced tocilizumab use. ICANS was lower in the OP group (6% vs 17%)
-
- Hospitalization within the first 30 days occurred in 43.8% in the OP group, with a mean of 1.1 IP days per pt vs 7.8 days for standard care
-
- The estimated total tocilizumab-related per-pt cost was $868 in the OP group vs $2,909 in the IP group
-
- Mean estimated cost per pt was $29,911 in the IP group vs $6,444 in the OP group
OP monitoring was safe, with no severe ICANS beyond G1 detected, and CRS events were managed effectively with OP interventions for G1. Tocilizumab use was significantly lower in the OP group (12.5% vs 42%; P=0.03), correlating with fewer severe CRS events.
- Takeaway: OP SUD of tec and tal with prophylactic dex and remote HaH monitoring is feasible, safe, and cost-effective. Compared to the IP, the OP group saw a reduction of 6.7 hospital days per pt and a 78% reduction in estimated cost per pt compared to IP observation. This approach could reduce pt burden and health care resource utilization
bsAbs Expand Options for NDMM and SMM
New findings highlight how bsAbs are advancing into earlier LOT. The first results from the phase 2 LINKER-SMM1 trial show that linvoseltamab may also offer clinical benefit for pts with high-risk SMM, suggesting a potential role for bsAbs in delaying disease progression and redefining early intervention strategies.
Additionally, phase 2 and early phase 3 data suggests that bsAbs like teclistamab and elranatamab, combined with standard agents, may be feasible and effective in NDMM pts. These findings point to a future where bsAbs may be given earlier in treatment—at least for a subset of pts; phase 3 trials are now under way.
Phase 2 Linker-SMM1 study of linvoseltamab for high-risk SMM
- Intervention: Linvoseltamab monotherapy with a 3-wk SUD schedule, followed by 200 mg dose in 28-day cycles (weekly for cycle [C] 1, every 2 wks for C2–5, and every 4 wks for C6–24)
- Population: 24 high-risk SMM pts per Mayo criteria (20-2-20; n=18) or PETHEMA criteria (n=6). Median age was 63 yrs
- Efficacy: ORR 100% (very good partial response or better [≥VGPR] 74%; ≥CR 37%)
- Safety: 92% of pts experienced ≥1 treatment-emergent AE (TEAE), and 38% experienced a G≥3 TEAE. Neutropenia was the only G≥3 hematologic toxicity. Infections occurred in 79% of pts and consisted of mostly low-grade respiratory tract infections. G3 infections were reported in 3 pts. CRS occurred in 10 pts (42%), and most events were G1 (a single G2 event after the 1 mg step-up dose). No ICANS observed and no pts discontinued treatment
- Takeaway: Linvoseltamab is highly active in SMM with a favorable safety profile. Linvoseltamab will be further investigated in this population
Phase 2 GMMG-HD10/DSMM-XX (MajesTEC-5) of tec
- Intervention: Tec (a BCMA × CD3 bsAb) combined with daratumumab-based induction regimens:
-
- Tec + daratumumab + lenalidomide (Tec-DR)
-
- Tec + daratumumab + lenalidomide + bortezomib (Tec-DVR)
Steroid use was limited to early induction cycles.
- Population: 50 transplant-eligible (TE) NDMM; median age was 58 years and 49 received treatment with Tec-DR or Tec-DVR as induction therapy
- Efficacy: During induction, partial response or better (≥PR) was achieved in 100% of pts across all arms, and all pts with available samples (n=46) achieved minimum residual disease (MRD) negativity at 10⁻⁵ and 10-6 (by next-generation flow cytometry and next-generation sequencing [NGS], respectively). Stem cell collection provided a median yield of 8.1 × 10⁶/kg, with only 1 mobilization failure
- Safety: Most pts (89.8%) experienced G3/4 TEAEs, mainly hematologic. No cases of ICANS were reported. G3/4 infections occurred in 34.7%; serious AEs in 53.1%. CRS occurred in 65.3% (all G1/2)
- Takeaway: Tec combined with daratumumab-based regimens (with or without bortezomib) as induction therapy in TE NDMM demonstrated unprecedented efficacy, including MRD negativity while maintaining the feasibility of stem cell mobilization and a manageable safety profile
Phase 3 MagnetisMM-6: part 1 of elranatamab + lenalidomide ± daratumumab
- Intervention: Elranatamab in combination with lenalidomide and/or daratumumab (EDR) to evaluate the optimal dose of EDR or ER for the recommended phase 3 dose for part 2
- Population: 34 transplant-ineligible (TI) NDMM pts treated with EDR, with a median age of 75 (range, 67–83; 38% male). Disease characteristics included 14% Revised Multiple Myeloma International Staging System (R-ISS) stage III, 24% with ≥50% bone marrow (BM) plasma cells (PCs), and none had EMD. ECOG 2 performance status in 3% and 24% considered frail by International Myeloma Working Group (IMWG) scoring
- Efficacy: The ORR was 97.3% (95% CI, 85.8–99.9); and 94.6% of pts achieved ≥VGPR, with a median TTR of 1.5 months. At the median follow-up of 7.9 months, most pts (32 of 34) remained on treatment
- Safety: All pts reported TEAEs; G3/4 events occurred in 95%
-
- CRS in 62% (all G≤2); 1 G2 ICANS case
-
- Hematologic AEs: 84% any grade, 78% G3/4
-
- Infections: 70% any grade, 19% G3/4; the most common were upper respiratory tract infection (22%) and E. coli urinary tract infection (11%)
-
- Serious infections: 1 G5 Candida pneumonia
-
- High rates of antimicrobial prophylaxis and immunoglobulin replacement, reflecting infection risk management
- Takeaway: EDR demonstrated a very high ORR with rapid and deep responses in older, frail, TI NDMM pts. Safety was broadly consistent with known risks of the regimen’s components, though infections and hematologic toxicity remain important concerns. The findings support continued investigation of elranatamab-based combinations in frontline MM
Tailored Strategies Improve Outcomes in High-Risk MM
New data explored optimized approaches for pts with high-risk or refractory features, including dual-bsAb combinations in EMD via the RedirecTT-1 study, the largest EMD study to date. This study reinforces the value of tailoring therapy intensity and combination strategy to disease biology.
Phase 2 RedirecTT-1 trial of tal + tec for EMD
- Intervention: Dual-bsAb regimen of tal (GPRC5D × CD3) and tec (BCMA × CD3). Dosing consisted of tal 0.8 mg/kg every 2 wks and tec 3.0 mg/kg every 2 wks, with SUD; pts could switch to every-4-wk dosing after C6 or after C4 with ≥VGPR
- Population: 90 pts with RRMM and EMD. The median age was 65, and 22% expressed high-risk cytogenetics. Patients had a median of 4 prior LOT (20% anti-BCMA CAR T, 9% prior bsAb therapy). 84% were triple-class refractory and 36% were penta-drug refractory
- Efficacy: The ORR was 79%, with ≥CR of 52%. In anti-BCMA CAR T–exposed pts, ORR was 83% and in bsAb-exposed pts 75%. The 9-month PFS and OS was 64% and 80%, respectively. Responses were durable, with most responders maintaining or deepening remission after switching to every-4-wk dosing
- Safety: Most pts (87%) experienced G3/4 AEs, and CRS emerged in 78% (all G1/2). Discontinuations occurred in 9% (5 due to G5 AEs, mostly infections), and 10 pts experienced G5 AEs, with 5 considered drug-related. Other AEs of note included:
-
- ICANS: 12% (G3, 1%; G4, 1%)
-
- Neutropenia: 62% (most common G3/4 AE)
-
- Taste changes (79%), skin-related events (69%), nail changes (56%)—all G1/2
-
- Infections: 79%, with G3/4 in 37% (majority within first 6 months)
-
- Hypogammaglobulinemia: 70%, with 87% requiring intravenous immunoglobulin (IVIG)
- Takeaway: Dual targeting with tal and tec resulted in high response rates, deep and durable remissions, and clinical outcomes comparable to CAR T therapy in heavily pretreated RRMM pts with EMD, a group with high unmet treatment need
Updated Trial Analyses Provide Insights Into Quadruplets for NDMM
Updated analyses were presented from key NDMM trials such as PERSEUS, CEPHEUS, IMROZ, and the phase 2 ISASOCUT study (subcutaneous [SC] isatuximab [isa] combined with bortezomib, lenalidomide, and dex). These analyses highlight pt-reported outcomes and safety findings, offering insights into regimen tolerability and response trends. These results show how pts fare in real-world and clinical trial settings and inform considerations for dosing schedules and management strategies.
PROs and safety from the phase 3 PERSEUS and CEPHEUS trials
- Approach: Assess pt-reported outcomes (PROs) and safety in pts who achieve MRD negativity vs those who remain MRD positive. PROs were evaluated at baseline and day 1 C1–8 and every 3rd C (CEPHEUS) or day 1 C1–C3, pre-ASCT, C5, C7 and then every 12 wks (PERSEUS). PROs included pts from the European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 (EORTC QLQ-C30)
- Population: 355/354 and 197/198 NDMM pts received daratumumab + bortezomib + lenalidomide + dex (D-VRd) and bortezomib + lenalidomide + dex (VRd), respectively, in PERSEUS/CEPHEUS
- Outcomes: MRD negativity was achieved in 75.2% pts (PERSEUS, D-VRd) and 47.5% (PERSEUS, VRd) pts, and in 60.9% (CEPHEUS, D-VRd) and 39.4% (CEPHEUS, VRd). The TEAE exposure-adjusted incidence rates (EAIR) per 100 pt-mo consistently favored pts who achieved MRD negativity vs MRD-positive pts in the DVRd arm in both trials. EAIR for G3/4 infections/infestations and G3/4 neutropenia were lower in pts who achieved MRD negativity vs MRD-positive pts. With D-VRd, mean change from baseline in EORTC QLQ-C30 global health status in pts who achieved MRD negativity vs MRD-positive pts in PERSEUS was 7.6 vs 3.3, and in CEPHEUS was 8.5 vs 4.2. Similar safety and PRO trends were seen with VRd in both studies
- Takeaway: Patients achieving MRD negativity stayed on treatment longer and maintained health-related quality of life despite prolonged treatment
Model-based assessment of monthly isa dosing
- Approach: Using data from the phase 3 IMROZ study, the use of isa 10 mg/kg every wk for 4 wks then every 2 wks, then every 4 wks at C18 onwards in combination with VRd vs VRd was compared via simulation to determine if an earlier switch to monthly isa dosing than the current 18-month reduces treatment burden without compromising efficacy
- Population: Simulation using modeling of PFS within the IMROZ trial framework with 357 evaluable NDMM pts
- Outcomes: Efficacy after switching to monthly regimen after
-
- 12 months (instead of 18)
- 60-month PFS rate: 76.2 vs 77.7%; HR, 0.543 vs 0.518
- 12.4% of pts expected to have shorter time to progression (TTP) by an average 4 months
- 12 months (instead of 18)
-
- 6 months (instead of 18 mo)
-
-
- 60-month PFS rate: 74.9% vs 77.7%; HR, 0.563 vs 0.518
-
-
-
- When restricted to pts with 3 mo-stable ≥VGPR status at 6 mo (78.6%), outcomes were comparable to 12-mo switch at population level. 5% of individuals could experience ≥8-month shorter TTP
-
- Takeaway: Isa dosing every 4 wks after 12 months in all NDMM pts or after 6 months in pts achieving stable ≥VGPR status did not compromise treatment efficacy vs switching after 18 months
Phase 2 ISASOCUT (IFM 2022–05) study of SC isa + bortezomib + lenalidomide + dex in NDMM
- Intervention: Fixed-dose SC formulation of isa in combination with bortezomib, lenalidomide, and dex (isa SC-VRd) as first-line therapy in TI NDMM pts
- Population: 74 pts with median age of 73. Approximately one third (34%) were >75 years. Almost a quarter (22%) of patents had high-risk cytogenetics (19% ISS stage 3, and 18% R-ISS stage 3)
- Efficacy: At 8 months, the primary end point (≥VGPR) was reached in 87.8% (95% CI, 78–94), and the ≥CR rate was 24%. MRD negativity rates were 35.1% at 10-5 and 27% at 10-6. No relapses were reported at a median 11.7 months follow-up and dose intensity of isa SC. Response maintained at ≥90% in most pts
- Safety: No new safety concerns emerged compared with SC isa. Infusion-related reactions 9.5% (mostly G1). Injection site reactions 27% (89.5% G1, remainder G2). Neurological AEs 47.3% (all grades, not further specified)
- Takeaway: The study met its primary end point, demonstrating consistent efficacy of isa in NDMM TI pts. These findings support isa SC-VRd as a new SOC for TI NDMM, offering a less intensive but longer induction regimen
CELMoD Therapies Expand Options Across Treatment Lines
CELMoDs, a class of next-generation immunomodulatory agents, are designed to enhance tumor cell degradation while stimulating the immune response. Agents such as iberdomide and mezigdomide demonstrated activity across multiple LOT, including in pts previously exposed to standard immunomodulatory imide drugs. These therapies offer potential accessibility for a broad range of pts due to manageable safety profiles and oral administration.
Phase 1/2 CC-220-MM-001 trial of iberdomide-daratumumab-dex in TI/deferred NDMM
- Intervention: Iberdomide, daratumumab, and dex (IberDd)
- Population: 75 adult pts with NDMM who have no plan for or are ineligible for autologous stem cell transplant (ASCT). 58.7% were ≥75 and 41.3% had high-risk cytogenetics
- Efficacy: ORR was 94.7% and deepening and durable responses with follow-up:
-
- ≥CR: 68%
-
- VGPR: 20%
-
- MRD negativity was achieved in 64% of pts overall; MRD-negative CR in 56%
-
- Median DOR and PFS had not yet been reached
- Safety: Safety profile consistent with prior analysis; no new safety signals
-
- G3/4 TEAEs: 97.3%
-
- Neutropenia was the most common hematologic toxicity in 78.7% of pts
-
- Infections: 52%
- Takeaway: With continued follow-up, iberDd continued to show notable efficacy, deep and durable responses, and manageable toxicity in TI NDMM, even in older and high-risk pts
Mezigdomide in novel combinations
- Intervention: Mezigdomide, an oral CELMoD, was evaluated in combination with dex (mezi-d) and novel agents (tazemetostat, the BET inhibitor BMS-986158, and the MEK inhibitor trametinib [TRAM])
- Population: 56 RRMM pts with progressive disease after their last regimen. Half (n=28) had received prior T-cell–redirecting therapies (TCRTs) including BCMA CAR T, GPRC5D CAR T, T-cell engagers (TCEs), and trispecific constructs. The remaining 28 pts had received non-TCRT regimens
- Efficacy: Mezi-d–based regimens induced marked proliferation and activation of CD4+ and CD8+ T cells, as well as natural killer (NK) and NK T–cell populations, regardless of prior TCRT exposure. Therapy promoted a shift toward an effector memory phenotype in CD8+ T cells, with reductions in naïve, central memory, and CD45RA+ effector-memory T-cell (TEMRA) subsets
-
- These immune dynamics mirrored those observed with mezi-d in non-TCRT populations, suggesting consistent immune activation capacity across heavily pretreated pts
- Safety: No new safety signals were reported specific to prior TCRT exposure
- Takeaway: Mezi-d–based regimens enhance T-cell and NK cell activation and proliferation in RRMM pts, including those previously exposed to TCRTs
Experimental Therapies Highlight Need for Clinical Trial Consideration
First-in-human and phase 1/2 data showcased innovative therapeutics. These included a novel trispecific antibody and CAR T constructs to other novel agents, including the antibody-drug conjugate (ADC) HDP-101, the BCL-2 inhibitor sonrotoclax, and dual-target CAR Ts.
These agents demonstrated encouraging efficacy and safety profiles that support continued clinical development. Importantly, the data highlights emerging questions about how best to combine or sequence these potent therapies.
Many of these trials enrolled pts who were heavily pretreated and refractory to multiple standard therapies. Efficacy in this difficult-to-treat population appears encouraging, underscoring the importance of clinical trial participation. Until optimal sequencing and combination strategies are defined, clinical trial participation remains essential.
Phase 1/2a trial of HDP-101
- Intervention: HDP-101, a novel ADC targeting BCMA carrying a synthetic amanitin payload, is being evaluated in adaptive dosing strategies, including split dosing and premedication (corticosteroids/antihistamines), to reduce treatment-related toxicity
- Population: 34 RRMM pts (median age 68.5, range 43–82; median of 7 prior treatment regimens, range 2–15). Prior therapies included BCMA-targeted CAR T and bsAbs in some pts
- Efficacy: Pharmacokinetics showed dose-proportional exposure with ~10-day half-life and minimal free amanitin in serum. But no clinical responses were observed below a dose of 90 μg/kg. Starting at 90 μg/kg, objective responses emerged:
-
- 90 μg/kg dose: 2/10 pts with PR
-
- 112.5 μg/kg dose: 2/3 ongoing pts with PR
-
- 100 μg/kg dose: 3/6 pts responded, including a stringent CR lasting >19 months in a pt previously treated with BCMA CAR T and GPRC5D/CD3 bsAb therapy
- Safety: HDP-101 was well tolerated overall, with no drug-related deaths, infusion reactions, or pulmonary or ocular toxicities
- Takeaway: HDP-101 showed a favorable safety profile up to 112.5 μg/ kg/cycle. Weekly or split dosing with premedication significantly reduced AEs. HDP-101 demonstrated early indications of efficacy at ≥90 μg/kg, including a durable, stringent CR in a heavily pretreated pt
Sonrotoclax + dex in pts with t(11;14)-positive RRMM
- Intervention: Sonrotoclax, a next-generation selective BCL2 inhibitor with higher potency and shorter half-life than venetoclax, was studied in combination with dex at 320 or 640 mg/day
- Population: 50 RRMM pts with t(11;14) translocation with a median of 3 (range 1–12) prior LOT; a high proportion were triple-class refractory
- Efficacy: The ORR was 64.3% in the 320-mg group and 80.6% in the 640-mg group. ≥VGPR was observed in 35.7% pts in the 320-mg group and 55.6% in the 640-mg group. Median TTR was 0.7 months in both cohorts. PFS was 6.6 months (320 mg) and 13.3 months (640 mg)
- Safety: The most frequent TEAEs were fatigue (35.7%) at 320 mg, insomnia (38.9%), and diarrhea (38.9%) at 640 mg (all G1/2)
-
- G≥3 hematologic toxicity, 7.1% (320 mg) vs 25.0% (640 mg)
-
- G≥3 infections, 21.4% (320 mg) vs 11.1% (640 mg)
- Takeaway: The all-oral combination of sonrotoclax + dex displayed a favorable tolerable profile with low rates of infection and hematologic toxicity and promising dose-related efficacy in pts with t(11;14)-positive RRMM. Additional combinations with sonrotoclax are being investigated
Phase 1 TRIgnite-1 Study of ISB 2001
- Intervention: ISB 2001 is a first-in-class BCMA × CD38 × CD3 trispecific TCE administered SC weekly across 9 dose levels (DLs; 5–2,700 μg/kg), with double SUD on days 1 and 4
- Population: 35 heavily pretreated RRMM pts with a median of 6 prior LOT, all triple-exposed, 71% penta-exposed, and 71% CD38-refractory. Prior BCMA-targeted therapy was received by 46% and 43% had prior T cell–redirected therapy (bsAbs and/or CAR T). High-risk disease features included cytogenetic risk (40%) and EMD (34%)
- Efficacy: ORR was 74% across all DLs. For all active doses ≥50 μg/kg, the ORR, CR rate, and VGPR rate were 79%, 30%, 64%, respectively; MRD negativity was 75%. Responses were rapid (median TTR 35 days) and durable (81% of responders ongoing, median DOR not yet reached). Pharmacodynamics confirmed consistent T-cell activation, supporting the intended mechanism
- Safety: No dose-limiting AEs were observed. AEs of note included:
-
- CRS: any grade, 69% (G1–2 only)
-
- Infections: any grade, 74%; G3/4: 29%
-
- Neutropenia: any grade, 51%; G3/4: 43%
-
- ICANS were rare (3%, G1 only)
- Takeaway: ISB 2001 demonstrated robust anti-MM activity and manageable safety in a heavily pretreated RRMM population, including pts with prior BCMA and CAR T/bsAb exposure. Efforts to optimize dose and schedule are under way
Phase 1 study of arlocabtagene autoleucel
- Intervention: Arlocabtagene autoleucel (arlo-cel), a GPRC5D-targeted CAR T-cell therapy, was evaluated in 2 dose expansion cohorts: 75 × 106 cells and 150 × 106 cells
- Population: 84 RRMM pts (24 pts at 75 × 106 dose, 26 pts at 150 × 106 dose) who were heavily pretreated with a median of 5 prior treatment regimens. All pts had prior proteasome inhibitor, immunomodulatory drug, and anti-CD38 therapy
- Efficacy: After a median follow-up of 19.9 months (75 × 106) and 24.0 months (150 × 106), the ORR was 92% (75 × 106) and 91% (150 × 106), respectively. The CR rate was 58% (75 × 106) vs 43% (150 × 106). The median DOR was 16.4 months (75 × 106) vs 13.6 months (150 × 106). Clearance of soluble BCMA was comparable across DLs
- Safety: G3/4 TEAEs occurred in 75% of the 75 × 106 group vs 69% in the 150 × 106 group. CRS occurred in 75% of the 75 × 106 group and 88% of the 150 × 106 group; all were G1/2 and resolved. ICANS occurred in 1 pt per cohort (G1 event, both resolved). On-target/off-tumor nail, skin, and oral toxicities were similar across cohorts, all G1/2. Overall safety profile remained consistent with prior reports
- Takeaway: Arlo-cel at both DLs demonstrated comparable efficacy and safety, suggesting a favorable benefit-risk balance in RRMM
Phase 1 trial of arlo-cel in pts with 1–3 prior regimens
- Intervention: Arlo-cel administered as a single infusion at the recommended phase 2 dose (150 × 106 CAR T-cells) following lymphodepleting chemotherapy
- Population: 31 RRMM pts with a median of 2 prior regimens; 29% received 3 prior regimens. High-risk features included 26% with del(17p), t(4;14), or t(14;16); 68% with 1q21 gain/amp; 32% with EMD
- Efficacy: There was a total of 24 evaluable pts; median follow-up was 15.8 months. ORR was 96% and CR rate was 63%. At follow-up, 15 of 24 responses were ongoing. Median PFS not reached; 12-month PFS rate was 74%. MRD negativity (at 10-5) was 75% (12 of 16 tested pts)
- Safety: TEAEs occurred in all pts; G3/4 in 87%. CRS occurred in 84% of pts (all G1/2, resolved). In addition, ICANS occurred in 10% (all G1/2, resolved). On-target/off-tumor effects included nail (39%), skin (42%), oral (42%) changes; all G1/2. Other neurotoxicity occurred in 6.5% (gait disturbance, ataxia, dysarthria; fully resolved) and infections occurred in 55% (all G1/2)
- Takeaway: Arlo-cel demonstrated deepening and durable responses in RRMM pts with 1 to 3 prior LOT, including those with high-risk disease. A phase 3 study of arlo-cel vs standard regimens has been initiated
Phase 1 TANDEMM trial of concurrent administration of BCMA and GPRC5D CAR T cells
- Intervention: Concurrent infusion of two CAR T-cell therapies—BCMA-targeted (MCARH125) and GPRC5D-targeted (MCARH109)—manufactured from a single apheresis and administered after lymphodepleting chemotherapy. Patients were treated at three DLs: BCMA-CAR alone (DL 0,150 × 106 cells) and BCMA-CAR + GPRC5D-CAR in varying doses (DL 1 [50 × 106 cells of MCARH109 and 150 × 106 cells of MCARH125] and DL 2 [150 × 106 cells of each])
- Population: 19 RRMM pts who had ≥3 prior LOT; 15 pts received treatment across DL 0 to 2. The majority (67%) in DLs 1/2 had prior BCMA therapy exposure (67%)
- Efficacy: ORR was 87% (13/15). By DL: 100% response at DL 0 (BCMA alone) and 78% response at DLs 1 and 2 (dual CAR). Median PFS was 20.1 months (95% CI, 17.5–not reached [NR]) for DL0 and 18.2 months (95% CI, 6.4–NR) for DLs 1 and 2. Antigen downregulation was linked to relapse; responders had enrichment of CD8+ memory T-cell populations. Expansion of one CAR product (BCMA) was reduced when both products were given together
- Safety: All pts experienced CRS; G2 CRS in 3 pts, but no G≥3 CRS was reported. G3 immune effector cell–associated hemophagocytic syndrome emerged in 2 pts (DL 0 and 2). No cases of non-ICANS neurologic toxicity were seen
- Takeaway: This proof-of-concept dose-escalation safety trial provided early evidence of the safety and efficacy of concurrent dual-target CAR T-cell therapy against BCMA and GPRC5D products manufactured from a single apheresis
New Biomarkers for Risk and Response
Emerging data highlighted new biomarkers that improve risk assessment and predict treatment outcomes, including MRD tracking via circulating tumor DNA. These findings potentially shift MM closer to an individualized treatment approach guided by immune and genomic profiling.
Two additional studies validated genomic-based models that better classify high-risk MM. The consensus model and new IMS/IMWG criteria aim to unify how high-risk disease is identified, improving trial design and individualized treatment strategies.
ClonoSEQ MRD testing from BM and circulating tumor DNA
- Approach: A retrospective analysis conducted at the Mayo Clinic to evaluate the clonoSEQ NGS assay for detecting MRD in peripheral blood (PB). Its diagnostic performance was compared with BM MRD and conventional biomarkers (M protein, free light chain ratio, EMD, and treatment response)
- Population: 149 PB plasma samples from 100 pts with secretory MM who had a known dominant clonotype identified in BM; pts included those at diagnosis, in remission, and with relapsed/progressive disease
- Outcomes: PB MRD negativity (10-6) correlated with deep clinical responses (CR and VGPR; P<0.001). In contrast, PB MRD positivity was associated with disease activity, including abnormal free light chain ratio (74%), detectable M protein (78%), and EMD (26%). Using BM MRD as a reference standard, PB MRD positivity had a specificity of 90.1%, positive predictive value (PPV) of 94.9%, sensitivity of 33.9%, and a negative predictive value (NPV) of 21.7%. In pts achieving ≥VGPR, PB MRD negativity showed 84% specificity, 93.6% PPV, 80.6% sensitivity, and 60% NPV
- Takeaway: PB MRD assessment with clonoSEQ offers a high-specificity, pt-friendly alternative to BM MRD testing in MM, with PB MRD positivity reliably indicating residual disease and PB MRD negativity strongly correlating with deep response.
CTCs as a critical biomarker
- Approach: An assessment of the prognostic impact of circulating tumor cells (CTCs) in NDMM and primary PC leukemia (pPCL) pts using flow cytometry, independent of established risk models
- Population: 2,364 pts with NDMM and 68 with pPCL from clinical trials and real-world settings across five European groups. Median age was 63, and 54% were TE. Induction regimens were predominantly triplet-based (88.9%), with fewer doublets (2.2%) or quadruplets (9.2%)
- Efficacy: Median CTC percentage was 0.017 and consistent across centers. Logarithmic stratification by CTC level identified five subgroups with distinct median PFS:
-
- ≤0.001%: 77 months
-
- 0.001%–0.01%: 51 months
-
- 0.01%–0.1%: 40 months
-
- 0.1%–1%: 31 months
-
- ≥1%: 16 months (similar to pPCL at 15 months; P<0.0001).
Higher CTC levels consistently predicted inferior PFS (HR 1.17, 95% CI 1.11–1.24, P<0.001), and the prognostic value remained consistent across subgroups, independent of R-ISS, 1q gain/amplification, induction regimen, or transplant status. In addition, CTC cutoffs between 0.01% and 0.1% reliably stratified pts in both trial and real-world settings and stratified pts with established risk factors: standard-risk pts with high CTCs had outcomes similar to those with high-risk cytogenetics but low CTCs (42 vs 36 months, P=0.48).
- Takeaway: Baseline CTC levels are a powerful independent prognostic biomarker in NDMM, and stratification by logarithmic CTC ranges identifies clinically meaningful subgroups and improves risk discrimination
High-risk genomic consensus model validation
- Approach: To validate, via an NGS panel, the revised IMS/IMWG high-risk genomic model for MM. High-risk is considered if pts present one of the following:
-
- del(17p)
-
- a TP53 mutation
-
- a biallelic del(1p32)
-
- a combination of t(4;14) or t(14;16) or t(14;20)/and either a gain of 1q or a monoallelic del(1p32)
-
- a combination of 1q gain and monoallelic del(1p32)
- Population: Between 2019 and 2024, 6,735 NDMM pts and 1,588 pts at first relapse were evaluated (from France). The PFS analysis included 2,703 pts, with ≥18 months follow-up
- Outcomes: High-risk prevalence was 23.9% and 36.7% at diagnosis and first relapse, respectively. When a high beta-2 microglobulin level (≥5.5 mg/L) in the context of normal renal function was added as a nongenomic risk factor, 31.4% of NDMM pts were classified as high-risk. Median follow-up was 35 months overall, and PFS was 29 months for high-risk NDMM and 51 months for non-high-risk (P<0.0001). For high-risk transplanted pts, PFS was 47 months vs 62 months for transplanted non-high-risk pts: (P<0.0001). The impact of individual abnormalities was as follows:
-
- del(17p), TP53 mutation, biallelic del(1p32), and combined lesions (t[4;14], t[14;16], t[14;20] with 1q gain or del[1p32]) all associated with significantly worse PFS than standard-risk pts
-
- With the accumulation of ≥2 high-risk criteria, median PFS falls dramatically (33 months with 1 vs 10 months with ≥2)
- Takeaway: This study validates the IMS/IMWG revised genomic definition of high-risk MM in a large real-world cohort. Roughly one third of NDMM pts met high-risk criteria, with clear evidence of significantly inferior PFS compared with standard-risk disease
IMS/IMWG risk-assessment criteria
- Approach: To evaluate the IMS/IMWG novel criteria for high-risk MM in a real-world prospective cohort
- Population: 332 consecutive NDMM pts in Greece were enrolled and prospectively stratified by novel IMS/IMWG high-risk MM criteria and existing staging systems (ISS, R-ISS, R2-ISS). 60 pts (18%) were classified as high-risk per the novel criteria (80% of these high-risk pts would not have been categorized as such by traditional tools)
- Outcomes: At a median follow-up: 37.5 months, pts identified as high-risk by the novel criteria had significantly worse outcomes than standard-risk pts:
-
- PFS was 25.5 months; doubled risk of progression/death (HR 2.15)
-
- OS was 30.0 months; 2.5-fold increased mortality (HR 2.50)
-
- TTP was not reached, but 85% had higher progression risk (HR 1.85)
Findings remained significant in multivariate analysis, confirming the independent prognostic utility of the criteria. Subgroup analyses demonstrated consistently inferior OS for high-risk vs standard-risk pts across age (>65, no high-dose melphalan with ASCT), performance status (ECOG ≥2), and treatment intensity (including quadruplets).
- Takeaway: The novel IMS/IMWG high-risk MM criteria identified pts with significantly adverse prognoses overlooked by established staging systems, suggesting that a substantial subset of NDMM pts classified as standard-risk by traditional tools have aggressive disease
Jointly provided by the MMRF and RedMedEd.
Support for this activity has been provided through sponsorships from Legend Biotech USA Inc., Pfizer Inc., and Sanofi and by educational grants from AbbVie Inc. and Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC.
For the last day of ASH, we’re taking a final look at some of the most exciting findings shared during Sunday’s sessions as well as late-breaking abstracts from this morning that highlight where myeloma research is headed next. Whether by investigating new regimens with already-approved treatments, or by developing unique, novel immunotherapies, the studies featured in our post today offer encouraging options for patients who need effective therapy options after relapsing.
Read the MMRF’s key takeaways below, and catch up on day one and day two.
For a deeper dive into the most important findings from ASH, join us for our Conference Highlights Webinar on December 17. Register here.
Combining the BCMA-targeting bispecific antibody Tecvayli (teclistimab) with Darzalex (daratumumab) leads to lasting responses for relapsed/refractory patients
In a phase 3 study, more than 500 patients who had relapsed after 1-3 prior therapies were given either one of these treatments:
- Tecvayli and Darzalex (TD)
- Darzalex, Pomalidomide, dexamethasone (DPd) or Darzalex, Velcade, and dexamethasone (DVd)
Patients taking the TD combination had a significant improvement in surviving without their disease progressing, compared to those who had either triplet therapy (83% vs. 29%). Disease did not progress in the TD group of patients for up to 3 years.
Side effects were mostly comparable between groups, with infections slightly occurring more in patients who were taking TD. Patients taking TD also experienced cytokine release syndrome that was mild mainly treatable.
Taken together, results from this late breaking abstract demonstrate that the combination of Tecvayli and Dazalex has the potential to be a new standard of care for patients who relapse early, especially on immunodmodulatory drugs like Revlimid or proteasome inhibitors like Velcade or Kyprolis.
New targets for bispecific and trispecific antibodies offer a path forward for relapsed/refractory patients with limited treatment options
Cevostamab: We heard new data on the bispecific antibody cevostamab as a treatment for relapsed/refractory myeloma, including patients with high-risk disease features and patients who have already received multiple other lines of therapies. Cevostamab targets a protein on myeloma cells called FCHR5—a different protein than the BCMA target used in Tecvayli, Lynozyfic,® and Elrexfio or the GPRC5D target used in Talvey (talquetamab). Therefore, it may help patients whose disease is resistant to those treatments. It is also given as an injection, rather than by IV, which provides greater convenience.
- In a phase 1b clinical trial, 58 RRMM patients, all of whom had previously received a median of five prior treatments, including other bispecific antibody therapies, were treated with cevostamab. Thirty-one percent had a very good partial response or better, and 20 percent achieved a complete response or better.
- An ongoing phase 1/2 trial is testing cevostamab in 27 patients with relapsed or refractory multiple myeloma who recently received CAR T-cell therapy. Most of the patients had high-risk features, such as certain genetic changes or cancer outside the bone marrow. Eighty-one percent had had a complete response, 95 percent of whom had no detectable cancer cells (MRD-negative). A year later, 93 percent were still cancer-free.
- Side efforts reported in both trials were mild to moderate.
IBI3003: IBI3003 is a new trispecific antibody that targets BCMA, GPRC5D, and CD3—combining the activity of two bispecifics in one drug. In a Phase 1 study of 28 patients who had received at least two prior treatments, all patients given the highest doses responded, including those with high-risk disease and those previously treated with BCMA or GPRC5D therapies like Tecvayli or Talvey. The most common side effects were fever and flu-like symptoms, and serious side effects were uncommon.
In patients who have exhausted treatment options targeting BCMA or GPRC5D with currently approved bispecifics or CAR T-cell therapy, these studies show that targeting other molecules on myeloma cells can offer therapeutic benefit.
Bispecific antibody combination show promises for relapsed/refractory patients with high-risk disease
High-risk multiple myeloma is a more aggressive form of the disease, often characterized by certain genetic changes, high levels of specific blood markers, or myeloma growing outside the bone marrow (extramedullary disease or EMD). These features can make the cancer harder to treat and more likely to return sooner, so patients may need stronger or more tailored treatment approaches. A combination of two bispecific antibodies — Talvey and Tecvayli — is showing particular promise for these patients.
Here’s a recap of the data:
- Across Phase 1B and Phase 2 studies in heavily pretreated relapsed/refractory multiple myeloma (including patients with EMD and other high-risk features), treatment with Talvey® and Tecvayli® produced overall response rates of ~75–90%, with ~50–60% achieving complete responses, with higher responses seen in patients with lower disease burden.
- For patients in both trials, expected side effects of Talvey and Tecvayli (CRS, low white blood cell counts, taste changes, skin changes, and infections) were common but generally manageable. However, it is important to note that in the first study, about 68 percent had serious infections, with many patients needing an infusion of donated antibodies (IVIG) to help strengthen the immune system. And, in the second, about 40 percent of patients had serious and life-threatening infections, and 6.7 percent died from these infections.
Despite these safety risks, this combination remains one of the most effective treatments reported for patients with this aggressive form of myeloma who otherwise have limited treatment options. It’s important to review the potential benefits and risks with your care team when considering this approach.
What’s Down the Road: In-vivo CAR T-Cell Therapy
Finally, in today’s late-breaking session, a phase 1 study explored the possibility of in-vivo CAR T therapy in a small group of 3 patients. Unlike traditional CAR T-cell treatment, which involves the removal of T-cells from patients and genetically reprogramming them in a lab, in-vivo CAR T can be accomplished inside the body, without the need for these steps or even bridging therapy. Simply put, a special infusion is given to patients that lets their own immune system generate CAR T-cells against BCMA.
In the study, patients experienced low-grade cytokine release syndrome which was treatable. Within one month, each of them so far has no detectable disease in the bone marrow.
While this study is early and involves only a small number of patients, it highlights the transformative potential of in-vivo CAR T therapy to simplify and broaden access to this powerful treatment approach. Notably, within the MMRF’s Myeloma Investment Fund portfolio, we have two in-vivo CAR T programs in development, an exciting step because eliminating the need for complex cell collection and manufacturing could make CAR T therapy more accessible to a much wider patient population.
That wraps up our coverage from ASH 2025. Be sure to stay connected with the MMRF for updates as these findings continue to move from clinical trials toward everyday care.
Today’s update from the 2025 ASH Annual Meeting highlights new and existing therapies that could improve care for patients at all stages of multiple myeloma. Read the key takeaways below, and be sure to catch up on the first day.
For a deeper dive into the most important findings from ASH, join us for our Conference Highlights Webinar on December 17. Register here.
For day 2, ASH presentations focused on existing therapies and asked two very important questions: 1) how well do they work, and 2) what can be done to improve them?
Answering this first question, new data covering the effectiveness of Isa-VRd (Sarclisa, Velcade, Revlimid, and dexamethasone) in different patient populations was presented, as well as updated data on Carvytki (cilta-cel), a CAR T-cell therapy that shows strong potential for providing long-lasting disease control for patients whose myeloma has relapsed.
To answer the second question, researchers presented on CELMoDs, a new class of oral therapies designed to enhance the immune response in myeloma. Studies showed encouraging results when CELMoDs were paired with different treatments across various stages of the disease.
New data support the use of Isa-VRd as a standard of care in newly diagnosed patients, with potential impact on quality of life
New data from a Phase 3 study suggest that Isa-VRd is more effective than Isa-Rd in treating older, high-risk NDMM patients who cannot undergo a stem cell transplant. As a reminder, Sarclisa is a monospecific antibody, similar to Darzalex.®
The study included over 200 patients aged 65–79 with high-risk disease features, such as certain genetic changes or high levels of a blood protein called β2-macroglobulin. Results showed:
- Thirty-one percent of Isa-VRd patients became MRD-negative compared to 13 percent of Isa-Rd patients.
- Isa-VRd patients were more than twice as likely to achieve MRD negativity.
- No new or unexpected side effects were reported.
Another study highlighted the impact of Isa-VRd treatment on quality of life. In over 600 newly diagnosed patients who were eligible for a stem cell transplant, Isa-VRd was linked to pain reduction, improved fatigue, and overall favorable quality of life compared to VRd alone.
IsaVRd is currently FDA-approved in the U.S. for NDMM patients not eligible for a stem cell transplant. However, some patients may access IsaVRd off-label, meaning it is used in ways not specifically approved by the FDA, based on a doctor’s judgment. Talk to your care team to see if IsaVRd may be an appropriate option for you.
Carvykti® keeps multiple myeloma under control for longer than standard 3-drug therapy
A large, Phase 3 study concluded that a single infusion with the CAR T-cell therapy Carvykti helped keep multiple myeloma under control longer than a standard 3 drug therapy, offering promise as an earlier treatment option for patients with relapsed/refractory myeloma. In this study, Carvykti was compared to two standard regimens: PVd (Pomalyst®, Velcade®, and dexamethasone) or DPd (Darzalex®, Pomalyst®, dexamethasone). Even after 2.5 years, three out of four patients treated with Carvykti still had their disease under control, compared to less than half of those treated with standard therapies. No new side effects were reported.
These data suggest Carvykti can provide safe and lasting disease control when used earlier in patients who have relapsed.
Can CELMoDS enhance immunotherapy in early disease and late relapse?
CELMoDs, which include the drugs iberdomide and mezigdomide, are oral treatments designed to help the immune system target and attack myeloma cells. CELMoDs work in a similar way as immunomodulatory agents, such as Revlimid® or Pomalyst®, but are designed to be more potent and effective. Oral treatments offer additional benefits in that they are easy to take and are available to anyone regardless of where they live.
One essential quality of CELMoDs is their ability to “rev up” the immune response. At ASH, many studies were focused on how well CELMoDs can enhance existing therapies. Whether combined with early-line therapies like stem cell therapy (ASCT) or later-line therapies like Elrexfio (elranatamab, a bispecific antibody), researchers are exploring whether CELMoDs can be effective across different stages of the disease—helping to deepen responses, achieve no detectable disease, and provide options for long-term treatment.
While CELMoDs are not yet available for routine clinical use, researchers are actively working to better understand their safety and effectiveness for potential future approval.
- For newly diagnosed multiple myeloma patients: A Phase 2 clinical trial in 120 patients tested different doses of iberdomide as maintenance therapy after a stem cell transplant. Iberdomide helped deepen responses in many patients, with about half achieving MRD-negativity, meaning they had no detectable cancer, and over 80% remaining cancer-free after two years. The most common side effect was low white blood cell counts, which sometimes led to infections; serious side effects were uncommon. Now that researchers have identified an effective and tolerable dose, Iberdomide can now be studied in larger trials.
- For patients who have relapsed following one to three prior lines of therapies: In a Phase 2 clinical trial, 30 patients with relapsed or refractory multiple myeloma were treated with a four-drug combination of iberdomide, Kyprolis®, Darzalex® and dexamethasone. All patients had previously received 1-3 prior lines of therapies, including Revlimid®. Ninety-two percent of patients responded to 4-drug combination treatment. Importantly, these patients were able to stay on iberomide alone for long-term treatment, with regular and ongoing MRD monitoring to make sure the disease stayed under control. While some patients had low blood counts, which could increase the risk of infection, it was manageable with medication or dose adjustments.
- For patients who have relapsed following two to four prior treatments: A Phase 1b study tested a combination of elranatamab, a BCMA-targeted bispecific antibody, and oral iberdomide in 22 patients with relapsed/refractory multiple myeloma who had received two to four prior treatments. After about six months, 91% of patients responded to treatment, and 46% had a complete remission or better. Most patients had side effects, usually mild or moderate, including fatigue, low blood counts, and diarrhea. Serious side effects were less common.
Stay tuned for tomorrow’s final dispatch from ASH 2025, where we’ll bring you even more exciting updates on groundbreaking research that is shaping the future of multiple myeloma care.
The 67th ASH Annual Meeting is underway in Orlando, where more than 30,000 experts have gathered to share the newest research in multiple myeloma and other blood cancers. The MMRF team is here on the ground, connecting with leaders in the field and bringing you updates as they happen.
Over the next three days, we’ll highlight promising new research and key insights shaping the future of myeloma treatment, many of which align with MMRF’s strategic research priorities and address critical unmet patient needs.
On Day 1 of ASH, studies focused on addressing the unmet needs of high-risk patients as well as finding ways to improve existing standards of care for all patients along their journey.
To dive deeper into these findings and additional updates from ASH, read more below and join us for our Conference Highlights Webinar on December 17. Register here to hear from leading experts and stay informed on the latest advancements.
As a reminder, many of the studies being presented, particularly oral abstracts, are early in nature. While the results shared at ASH can be very strong and encouraging, much larger trials will need to be completed to confirm these initial findings, especially in patient populations that are representative of the real-world patient population in the U.S.
New CAR T Treatment Tested in High-Risk, Newly Diagnosed Patients
High-risk multiple myeloma is a more aggressive form of the disease, often marked by certain genetic changes, high levels of specific blood markers, or myeloma growing outside the bone marrow. A real-world study presented today reinforced that these high-risk features can make myeloma harder to treat and more likely to return sooner, highlighting the need for closer follow-up or additional therapy for these patients.
A small, phase 1 study presented early data on a potential new treatment for high-risk newly diagnosed patients who are not eligible for a stem cell transplant. Sixteen patients received an infusion of the CAR T-cell therapy eque-cel. All had a deep response within the first month, including complete responses and minimal residual disease (MRD) negativity, meaning no detectable myeloma using very sensitive tests. Most patients stayed MRD-negative for two years, keeping disease under control during this follow-up period.
Common side effects included CRS (cytokine release syndrome), low blood counts, and infections, but no cases of ICANS (Immune Effector Cell-Associated Neurotoxicity Syndrome) or other neurological toxicity were reported. While these results are promising, more patients and longer follow-up time are needed to confirm the benefits of eque-cel as an early treatment option for this high-risk group.
Improving Treatment Strategies for Newly Diagnosed Patients
New research shows real progress in treating people who are newly diagnosed with multiple myeloma. Three studies presented today look at different treatment approaches, including updated drug combinations, a CAR T-cell therapy that can be made in one day instead of weeks, and an immune-based treatment typically used for treating patients with relapsed/refractory disease.
- KRd leads to more complete and deeper responses than VRd in NDMM patients
For newly diagnosed patients who may not be able to tolerate four drug treatments, what is the best way to ensure three drug combinations are still effective? One Phase 3 study of 250 patients compared two common triplet combinations for newly diagnosed multiple myeloma patients to see what works better: KRd (Kyprolis®, Revlimid®, dexamethasone) vs VRd (Velcade®, Revlimid®, dexamethasone). Both combinations worked well, but KRd led to more complete and deeper responses than VRd (see table below). This means that M protein levels were absent from the blood and plasma cells were not detectable in the bone marrow.
| Response Measure | KRd | VRd |
|---|---|---|
| Overall Response Rate (ORR) | 94% | 91% |
| Complete Response (CR) | 71% | 53% |
| MRD-Negative | 31% | 18% |
Side effects were similar between the two treatments: serious side effects occurred in about 7 in 10 patients on KRd and 6 in 10 on VRd, the most common of which were low white blood cell counts and infections. Patients treated with KRd had fewer nerve-related problems than VRd, but slightly more heart-related effects, while patients treated with VRd had more neuropathy than patients treated with KRd.
- “Next-day” CAR T-cell therapy shows strong results and may open door for shorter wait times
A Phase 1 clinical trial tested a new CAR T-cell therapy for newly diagnosed patients that targets two proteins on the surface of myeloma cells—BCMA and CD19—instead of just one. All patients responded, and 97% had a complete response. While CRS was common side effect, it was manageable with treatment, and no patients had any serious nerve-related side effects. Importantly, this CAR T-cell therapy can be made overnight, rather than the typical timeframe of weeks seen with other CAR T therapies, potentially making the treatment available to those who need it sooner. - Bispecific treatments brought in earlier along the patient journey
With the hope of making therapeutic responses last longer, bispecific antibody treatments used for treating relapsed/refractory patients are now being tested as an alternative to traditional chemotherapy and stem cell transplant for newly diagnosed patients:- One such treatment is Lynozyfic.™ In an early Phase 2 study, all 14 people treated had a deep response, and everyone tested was MRD-negative at six months. Side effects of Lynozyfic included infections, rash, bone pain, or low blood counts, which were mild for most patients.
- Another is a combination of Tecvayli® and Darzalex.® Both Tecvayli and Darzalex are FDA-approved for patients with relapsed/refractory multiple myeloma; Darzalex is also approved for newly diagnosed patients when used in certain combination treatments. In a Phase 2 study of 37 newly diagnosed multiple myeloma patients ages 65 and older patients received this “all-antibody” combination as their initial therapy. Seventy-eight percent had a very good partial response or better after four treatment cycles, and eventually all patients responded. More than half (51%) had no detectable myeloma six months after starting treatment, and no patients experienced disease worsening. Common side effects included low blood counts and infections, which were mostly mild to moderate.
New CAR T-cell therapy shows promise in treating relapsed/refractory patients with limited options
A Phase 2 study tested anito-cel, a CAR T-cell therapy designed to more easily attach to myeloma cells using a small “hook”—a feature that may help reduce certain side effects seen with other CAR T-cell therapies. Key findings from 117 patients included:
- 97 percent responded, and 68 percent reached a complete remission or better, usually within about one month.
- Most patients remained cancer-free at the one-year mark.
- The most common side effects were low blood counts and mild to moderate cytokine release syndrome, which typically resolved quickly. Serious infections and neurological problems were uncommon.
These data suggest that anito-cel may offer strong, long-lasting control of myeloma, with manageable side effects, even for people whose disease has stopped responding to many other treatments.
We’re hopeful about the progress shared today at ASH—and we’ll be back tomorrow with more updates for the myeloma community.
People living with multiple myeloma often relapse after several lines of therapy. When this happens, it can feel like options are running out. The recent approval of Lynozyfic (linvoseltamab-gcpt) is an important step forward in expanding treatment option for patients.
Lynozyfic was approved in July 2025 for adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy.[1] Here are some important details about Lynozyfic:
- Lynozyfic is a bispecific antibody—a type of treatment that helps cells in your immune system find and attack myeloma cells.
- Lynozyfic works in a way that’s similar to other bispecific treatments like Tecvayli and Elrexfio—they all target a protein called BCMA, which is found on myeloma cells. This makes it easier for the immune system to recognize and attack those cancer cells.
- Bispecific antibodies like Lynozyfic are generally more accessible, because they don’t require the individualized manufacturing process or lengthy preparation time associated with CAR T-cell therapy.
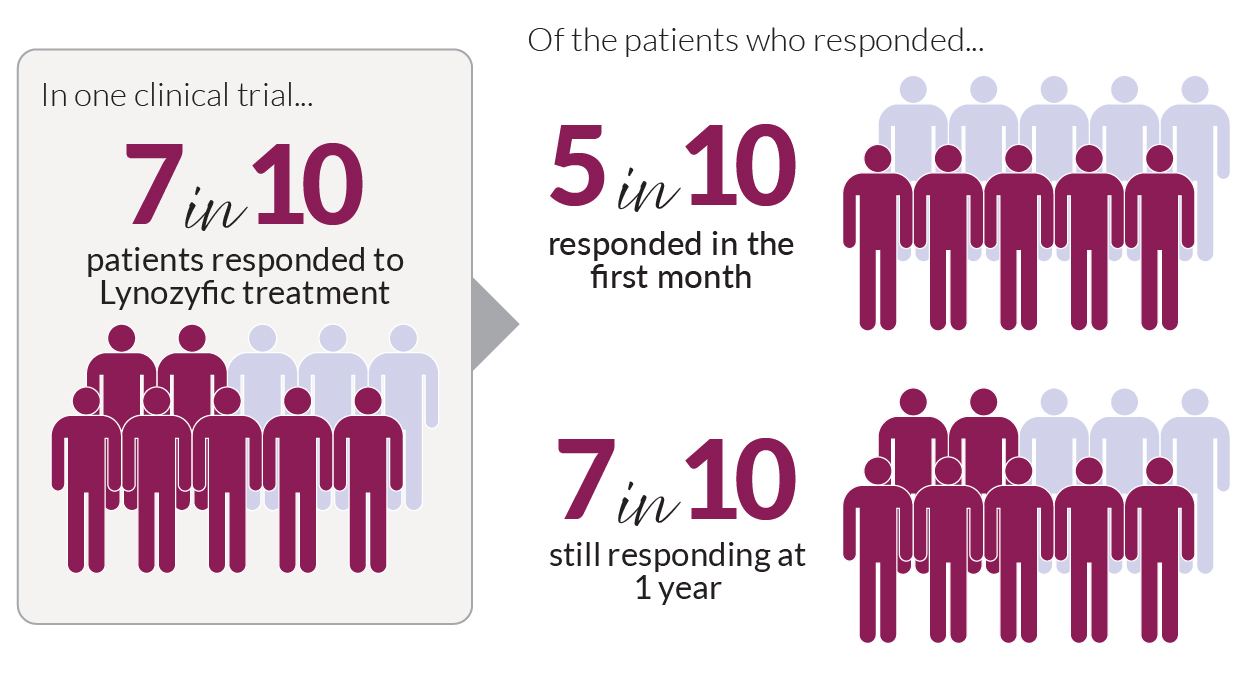
A clinical trial with myeloma patients who had received at least four prior treatments showed that 70% of them responded to Lynozyfic treatment. Of these individuals:
- Half of the patients responded within 1 month of treatment
- For 70%, the response has continued for a year (see figure below).
[1] Including a proteasome inhibitor (such as Velcade, Kyprolis, or Ninlaro), an immunomodulatory agent (such as Revlimid or Pomalyst), and an anti-CD38 monoclonal antibody (such as Darzalex or Sarclisa).
Lynozyfic is given through an intravenous infusion:
- Treatment starts with two step-up doses, which are small doses that gradually increase
- After each step-up dose, you’ll have to stay in the hospital for 24 hours to be monitored for side effects
- After you’ve received the step-up doses, you’ll receive the standard dose once a week for a total of 12 doses without any hospital stay
- After the first 12 full doses, you’ll receive one full dose every 2 weeks for 5 weeks
- If you’re responding, your doctor may decide to drop your treatment to once every 4 weeks; if not, your treatment will continue on the every-2-weeks schedule
Like many myeloma treatments, Lynozyfic can cause side effects. The most common is cytokine release syndrome, which can cause fever, chills, or trouble breathing. If these signs occur, it’s important to report them to your care team right away.
Several options are available for avoiding or reducing side effects. Neurologic problems—in particular, immune effector cell-associated neurotoxicity syndrome (ICANS)—can also occur, but they are not as common as CRS.

Because of the side effect risks, Lynozyfic is only available through a special safety program. If you receive Lynozyfic, you’ll be given a wallet card to remind you and your care providers of what to watch for.
If you’re a caregiver for a myeloma patient, make sure the patient carries their wallet card and watch the patient for symptoms. If any develop, report them to the care team right away.
As with any myeloma treatment, it’s important for you and your caregiver to have open conversations with your care team about whether Lynozyfic is a good fit. If you’ve already been through several treatments, Lynozyfic offers a new chance at controlling your myeloma.
Interested in learning more about Lynozyfic? Talk to an MMRF patient navigator at the Patient Navigation Center at 1.888.841.MMRF (Monday through Friday, 9:00 AM to 7:00 PM ET) or email [email protected].
Additional Resources
- Regeneron. Lynozyfic (linvoseltamab-gcpt) Patient Support.
- MMRF. Fast Facts in Myeloma. Bispecific Antibodies for Multiple Myeloma: What You Need to Know.
- MMRF. MMRF Patient Toolkit. Immunotherapy.
- MMRF. MMRF Patient Toolkit. Multiple Myeloma Treatment Overview.
- MMRF. MMRF High-Impact Topic Video. Bispecific Antibodies.
Less than a year after enrolling its first patient, the Horizon One clinical trial already has important wins to celebrate: Not only is it pioneering important research for patients with relapsed and refractory multiple myeloma, but it’s enrolling a patient population that’s far more representative of the disease than other trials to date.
The Horizon Clinical Trials program, which is run by the MMRF’s Multiple Myeloma Research Consortium® (MMRC®), is innovative by design, starting with the fact that it’s aiming to optimize treatments for patients with significant unmet need. Horizon One is enrolling patients with relapsed and refractory multiple myeloma, while the forthcoming Horizon Two trial will enroll high-risk, newly diagnosed patients.
Unlike a traditional clinical trial, Horizon is what’s known as an adaptive platform clinical trial. This unique design allows investigators to test several treatments at the same time and has built-in flexibility to add or remove therapies in real time—all with the goal of generating data and insights at an accelerated pace.
Speed is especially important for the patients in Horizon One. While there are currently more than 15 FDA-approved drugs for the disease, the devastating reality is that most patients will relapse when the disease becomes resistant to a therapy. Others may eventually become refractory (unresponsive) to many kinds of treatment.
“This patient population needs more options, and they need them fast,” said the MMRF’s Chief Medical Officer Hearn Jay Cho, MD, PhD. “These patients and their providers still face complex questions about what treatments will be most effective and in what order. Horizon is designed to answer those critical questions that other types of trials can’t.”
Horizon is innovating in another way: by enrolling a study population that is representative of multiple myeloma patients across the United States. In doing so, the Horizon trial can generate new insights and answer questions that impact all patients.
Historically, clinical trials in multiple myeloma have not been as inclusive.
About 20 percent of multiple myeloma patients in the U.S. are Black, but those patients have been significantly underrepresented in clinical trials. In fact, one analysis of multiple myeloma trials conducted over a 13-year period found that only four percent of enrolled patients were Black.
The average age of a multiple myeloma diagnosis is 69, and two-thirds of people diagnosed with the disease are 65 years or older. But studies have found that these patients are also left out of research. One analysis found that patients over the age of 70 are half as likely to enroll in multiple myeloma clinical trials, and another found that nearly two-thirds of trial participants over a 16-year period were under the age of 65, not at all representative of the disease’s true age demographic.
But the MMRC’s Horizon trial is working to reach the broadest myeloma population—and succeeding.
As of now, more than 25 percent of patients enrolled in Horizon One identify as Black. Seventy-one percent of patients are 71 or older, with more than 20 percent between 81 and 90 years old, percentages that are rarely, if ever, seen in other myeloma trials.
Horizon One is also working to enroll across a mix of geographical locations, including in rural areas and at 25 community hospitals and clinics. In fact, the MMRC Horizon One trial recently opened the first-ever myeloma clinical trial in Flint, Michigan.
Meeting these impressive goals has required considerable collaboration that the MMRC—with its vast network of 13 major medical centers and researchers and a leadership team with decades of expertise and industry connections—is uniquely positioned to facilitate.
Working closely with the MMRC’s Health Equity Advisor Dr. Craig Cole of Karmanos Cancer Institute, the Horizon team has surveyed MMRC sites, collecting data and insights about why patients enroll and, importantly, why they don’t. Dr. Cole and the MMRC team have also brought all 13 MMRC sites together to share learnings and wins.
“Clinical trials have been exclusionary to older people, people of color, and people who live far from academic medical centers. We can enroll those populations by knowing why patients don’t consent to the clinical trial,” Dr. Cole said. “We’re making cultural change. The MMRC sites are sharing stories and ideas and thinking outside the box about how we can improve what we’re doing.”
Many of the trial’s wins, like the launch in Flint, came out of simply having intimate, one-on-one conversations out in the community.
“I did a church basement talk in Flint at an African American church, and I gave my awareness talk about what myeloma is,” Dr. Cole said. “And this little old lady said, ‘You need to bring clinical trials to us. I don’t want to hear about myeloma and all your fancy drugs that you give in Detroit.’ I took it back to the MMRC and said, ‘A little old lady told me to bring a clinical trial to Flint, and I think we really need to do that.’ And the MMRC leadership made that happen. Now, that little old lady can go down the street, get her bispecific, go home, and hang out with her grandchildren.”
Understanding that cures don’t come out of silos, the MMRF and MMRC have a long history of making their learnings and data available to the wider multiple myeloma field. Looking forward, the Horizon trial investigators plan to share their experiences and findings for other researchers, pharma, academic medicine, and others to learn from.
“We’re so proud to conduct trials like Horizon that will help us better understand the disease across a representative population and regardless of where someone lives,” said MMRF’s Senior Vice President of Clinical Research and Regulatory Affairs Katie Wozniak. “That’s how we’ll improve care and find cures for everyone diagnosed with multiple myeloma.”
The MMRF is excited to share that the FDA has approved Blenrep (belantamab mafodotin) combinations for multiple myeloma patients who have relapsed and have received at least two prior lines of treatment. The announcement was eagerly anticipated, especially after the FDA took some time to weigh the data presented at July’s Oncology Drug Advisory Committee (ODAC).
This is encouraging news for our community. As an IV infusion like Sarclisa or Kyprolis, Blenrep provides an additional off‑the‑shelf treatment option that can help more people access care from their doctor’s office or cancer center. Also, unlike CAR T or bispecific therapy, patients can begin treatment without hospitalization needed to manage certain side effects like CRS or ICANS.
What to Expect with Blenrep
Clinical trial data showed how safe and effective Blenrep (in combination with Velcade and dexamethasone) was for patients compared to Darzalex, Velcade, and dexamethasone. Here were the highlights:
- Improved responses: Adding Blenrep to standard treatment helped patients live almost three times longer without their cancer getting worse.
- Manageable side-effects: The most common serious side effect with Blenrep was eye-related problems (such as blurred vision or dry eyes), which were usually manageable with dose changes, eye drops, or treatment breaks, but often required close monitoring. Other side effects seen were typical for myeloma therapies (like low blood counts and tiredness), and doctors monitored patients regularly to help manage these issues.
Next Steps for Patients
Now that Blenrep is available, it is important to have a conversation with your care team if this treatment could be a viable option for you. Here are some questions to ask that help start the discussion:
- How does Blenrep compare with the other treatments I’m considering?
- What side effects should I watch for, especially related to my eyes, and how will we monitor and manage them throughout treatment?
- How often will I need to come in for infusions and eye exams, and what will my schedule look like day-to-day?
- Will this treatment affect my daily activities, like driving, working, or traveling, and what support resources are available?
In addition to your care team, the MMRF Patient Navigation Center can offer support in answering these questions and give one‑on‑one guidance tailored for you. The MMRF Education Hub also offers additional materials to help better understand treatment options and navigate you along your myeloma journey.
In late 2024, the MMRF launched the Horizon Clinical Trials Program through the Multiple Myeloma Research Consortium® (MMRC), a collaborative research network of leading myeloma treatment centers. Horizon uses an innovative trial design called an adaptive platform, which makes it possible to test multiple therapies at the same time to determine the best combination, sequence, and duration of treatment to get the best outcomes for patients. The adaptive design also allows researchers to work together and move faster than they can in a traditional clinical trial model.
For the first trial within the Horizon program (Horizon One), we are enrolling patients with relapsed and refractory myeloma across 13 sites to study dosing approaches with Tecvayli® (teclistamab), a bispecific antibody treatment. The second platform trial of the program, Horizon Two, is scheduled to launch soon and enroll high-risk, newly diagnosed patients.
Horizon Two will reimagine how we treat high-risk newly diagnosed multiple myeloma. We spoke with Dr. Sham Mailankody of Memorial Sloan Kettering (MSK) Cancer Center about what patients can expect — and why this moment marks a turning point in myeloma research.

Dr. Sham Mailankody, Memorial Sloan Kettering (MSK) Cancer Center
Why do people with high-risk myeloma need different treatment options?
Over the past 25 years, treatments for multiple myeloma have improved a lot, especially for patients with what we call “standard-risk” disease. But people with high-risk myeloma haven’t seen those same benefits. About 1 in 4 newly diagnosed patients fall into this high-risk category, and unfortunately, these patients either do not respond to their initial therapy, or relapse within two years — whereas most patients are in remission for at least four or five years after their first therapy.
What is the Horizon Two trial, and how is it different from other studies?
Horizon Two is a national clinical trial created specifically for people who are newly diagnosed with high-risk myeloma. In the past, this group was often left out of research. The trial is designed to be flexible and patient-friendly. It allows multiple treatment options to be tested at once, and new ones can be added over time. Even patients who have already started their first cycle of treatment may still qualify. This approach helps us learn faster and include more people in the process.
What kinds of treatments are being tested in Horizon Two?
We’re looking at advanced treatments called immunotherapies — including CAR T-cell therapy and bispecific antibodies. These therapies have already worked well for patients with advanced or relapsed myeloma. Now we’re testing whether using them earlier, right after diagnosis, might lead to stronger and longer-lasting responses.
Will the trial consider how treatment affects patients’ quality of life?
Absolutely. Some treatment arms in the trial will explore time-limited therapy, meaning patients could stop treatment after a certain amount of time if they’re doing well. We’re also collecting direct feedback from patients and advocates to guide future parts of the study. Better treatment doesn’t just mean controlling the disease — it means improving how patients feel and live.
How is Horizon Two making it easier for more people to take part in research?
Horizon Two is available at many locations across the country — not just at large academic hospitals, but also at regional and community centers that work closely with myeloma experts. For example, patients don’t have to travel to Manhattan to take part in the trial at MSK; there may be a site closer to home. This setup helps reduce travel time and costs, making it easier for people, including those in rural or underserved areas, to access cutting-edge research and care.
How important is collaboration in making a study like this happen?
It’s absolutely necessary. Horizon Two is the result of years of collaboration through the MMRF and MMRC. It brings together top cancer centers, patient advocates, drug companies, and government regulators. Everyone plays a role: from designing the trial to enrolling patients. This kind of teamwork is what makes real progress possible.
What does success look like for Horizon Two?
Our biggest goal is to close the gap in outcomes between high-risk and standard-risk patients. If we can do that, we may one day no longer need the term “high-risk.” That would be a huge step forward for more effective treatments and for a more equitable future for everyone living with myeloma.
Between declining government funding for research and a difficult drug development arena marked by higher costs, greater risk, and more competition for less capital, multiple myeloma is at a crossroads. Cures are within reach, but to realize them, the community needs to confront these challenges head-on—something the Multiple Myeloma Research Foundation (MMRF) is uniquely poised to do.
One of the most important ways we’ll push the field forward now is through the Myeloma Investment Fund (MIF), our venture philanthropy arm. Over the last six years, the MIF has helped advance six treatments to clinical trials, invested $23 million in 20 companies, and generated a two-to-fourfold return on its initial investment in three of those companies.
Now, the MIF is scaling up when myeloma needs it most and will soon become a self-sustaining, evergreen fund— reinvesting all the returns it generates into new companies and ideas.
The MIF is the first and only mission-driven philanthropic venture fund focused on accelerating a cure for multiple myeloma. The MIF provides biotechnology companies with financial and strategic support, access to data, and connections to investors, researchers, and pharma representatives.
Not only that, but the MIF leverages the MMRF’s vast expertise and its more than 25 years at the forefront of myeloma research.
“We join the boards of many of these companies and play an important advisory role,” said MIF Managing Director Stephanie Oestreich, PhD, MPA.
The companies the MIF invests in are developing some of the most transformative potential treatments in myeloma—with a special focus on relapsed patients who have run out of options.
“An estimated 70 percent of new therapeutic targets are being developed by venture-backed companies, and only 30 percent are with pharma,” Oestreich said. “Venture capital like the kind the MIF provides is critical to move tomorrow’s innovations forward.”
MIF investments provide funding at a pivotal point in the drug development process, when early breakthroughs are at risk of stalling because they lack funding or direction. By stepping in at this phase, the MIF helps bridge the gap between research and real-world application.
“At a time when early-stage funding is harder to secure than ever, the MIF’s early support is essential—helping companies attract more investors and ensure that the best new ideas in myeloma don’t get left behind,” the MMRF’s President and Chief Executive Officer Michael Andreini said.
MIF-backed companies are working on some of the most exciting new technologies and developments today: next-generation CAR T-cell designs and treatments (such as in vivo CAR T, which engineer T-cells in a patient’s body instead of a lab), natural killer cell–directed therapies, antibody-drug conjugates (which have revolutionized treatment of other cancers), and more. Our portfolio also includes companies investigating novel ways to improve patients’ immune response to better recognize, attack, and kill myeloma cells.
The companies the MIF invests in are also highly regarded. In fact, in September, two such companies—Umoja and Stylus Medicine—made Endpoints’ annual list of the 11 most exciting biotech startups of 2025. Endpoints is one of the most prestigious news outlets covering biopharma, and its annual Endpoints 11 list is highly anticipated in the field—representing “the best and the brightest in biotech.”
Andrew Scharenberg, MD, the co-founder and CEO of Umoja Biopharma, which the MIF invested in at the start of 2025, said that having the backing of the MIF and MMRF is crucial in more ways than one.
“Because the MIF and MMRF are trusted by doctors and scientists, their support helps open doors,” he said. “When others see that the MIF believes in what we’re doing, it gives them confidence that our work is promising and based on solid science. That support helps us move faster and bring new treatments to patients sooner.”
The final day of IMS 2025 brought a powerful close to the meeting, underscoring how far the field has come in advancing personalized care for myeloma patients. From promising new drugs to smarter ways of tailoring treatment using biomarkers, today’s presentations made it clear: The future of myeloma therapy is not just about more options—it’s about better-matched options.
Researchers are now focusing on how to deliver the right treatment to the right patient at the right time, using tools like genetic profiling and immune markers to guide decisions.
Read on for what stood out to us.
New Treatments on the Horizon for Patients at All Stages of Disease
Day four showcased a pipeline of innovative treatments poised to transform myeloma care, from precision-targeted therapies that could redefine treatment standards to enhanced delivery methods that are more convenient for patients.
- On-body-injector for Sarclisa (isatuximab): For myeloma patients, having options that reduce discomfort and fit more easily into daily life is critical. In a phase 2 and phase 3 trial, patients with relapsed or refractory disease tried Sarclisa given through a small wearable injector instead of the traditional IV drip. Surveys looking at quality of life were given to these patients, who reported less pain, less discomfort, fewer side effects than expected, and meaningful time savings compared to IV treatment. Overall, more patients were satisfied with the on-body injector and said they would recommend it to others.
- Iberdomide: Iberdomide is part of a new class of drugs called CELMoDs. CELMoDs are related to IMiDs like Revlimid and Pomalyst, helping to boost the immune system’s ability to fight myeloma. Like IMiDs, they are also pills. In small clinical trial of 75 newly diagnosed patients who were ineligible for transplant, a combination of the oral CELMoD iberdomide with Darzalex (daratumumab) and dexamethasone (IberDd) led to responses in about 95% of people treated. Over 2 years, more patients in this study began to experience deeper responses. Side effects of the IbderDd regimen were manageable, and included infections, diarrhea, and rashes.
- Sonrotoclax: Sonrotoclax is a new oral drug designed to target a specific genetic change called t(11;14) that is found in approximately 20% of patients, and is similar to Venclexta (venetoclax), which is sometimes used off-label for patients with this genetic feature. In a phase 1/2 trial of 50 patients with relapsed/refractory myeloma, 81% responded to a combination of sonrotoclax and dexamethasone. Responses were often seen within three weeks and lasted over a year for many patients.
- HDP-101: HDP-101 is a new anti-BCMA antibody-drug conjugate (ADC) that delivers a powerful toxin directly to myeloma cells while sparing other healthy cells. In a phase 1/2 study of 42 relapsed/refractory patients, HDP-101 was generally safe, especially when given in split doses with premedication to reduce side effects like low platelets and liver changes. There was no evidence of eye-related side effects due to the drug; eye-related side effects are a common side effect with belantamab mafodotin, which is also an anti-BCMA ADC. About 50% of patients who were treated at higher doses responded to this therapy. Additional studies are underway to determine the dose that best balances safety and effectiveness. In this study, patients had already gone through about 7 different therapies, with some trying as many as 15. Even after many relapses, having new treatment options available is very encouraging. It means there is still hope and progress for patients who need more choices.
Strengthening Maintenance Therapy
As doctors work to improve myeloma treatments, researchers are studying whether using more than one treatment for maintenance therapy can help lower the chance of relapse. In a phase 3 study, patients who took a combination of Darzalex and Revlimid for two years did much better at keeping myeloma to very low, almost undetectable levels, compared to patients who took Revlimid alone. Just as important, the side effects were mild and very similar to what patients usually experience with Revlimid by itself. As discussed on day 3, these studies are especially important for patients who may have a higher risk of their disease returning quickly.
The Increasingly Important Role of Biomarkers
During the last day of IMS, we also heard updates on the growing role of biomarkers—biological signs in the body that help doctors better understand each patient’s type of myeloma. These discoveries build on earlier research from the MMRF’s CoMMpassSM study, which is one of the most important studies ever conducted to better understand new targets for drug development and define which patients are more likely to have aggressive disease. Researchers across the field continue to leverage CoMMpass data.
- Biomarkers to predict risk: Earlier this year, experts introduced a new way to define high-risk multiple myeloma, a more aggressive form of the disease that is harder to treat. Two studies shared today showed that this new definition is not only accurate in predicting worse outcomes among high-risk patients, but it more effective than traditional staging systems, providing strong support for the use of this new definition.
- Biomarkers to guide treatment planning: Biomarkers are also helping doctors choose the best treatments for each patient. Several presentations focused on using biomarkers to predict how well a patient might respond to newer treatments like CAR T-cell therapy and bispecific antibodies, including who may be more likely to experience certain side effects. There’s also progress in using blood-based tests for monitoring, which could reduce the need for more invasive procedures like bone marrow biopsies.
These updates reflect a powerful shift in how myeloma is being treated—from relying solely on standard drug regimens to using a deeper understanding of each patient’s unique disease to guide care. As more targeted therapies and biomarker-driven tools move closer to routine use, the path forward looks increasingly personalized, offering new hope for better outcomes and a higher quality of life for patients at every stage of their journey.

