News & Events
Carvykti (ciltacabtagene autoleucel): Expanded Options for Patients With Relapsed or Refractory Myeloma
Carvykti (ciltacabtagene autoleucel) was recently approved for use in myeloma patients who have had at least one prior treatment that didn’t work or stopped working and who no longer respond to Revlimid. Previously, Carvykti was only an option for myeloma patients who had received at least four prior treatments.
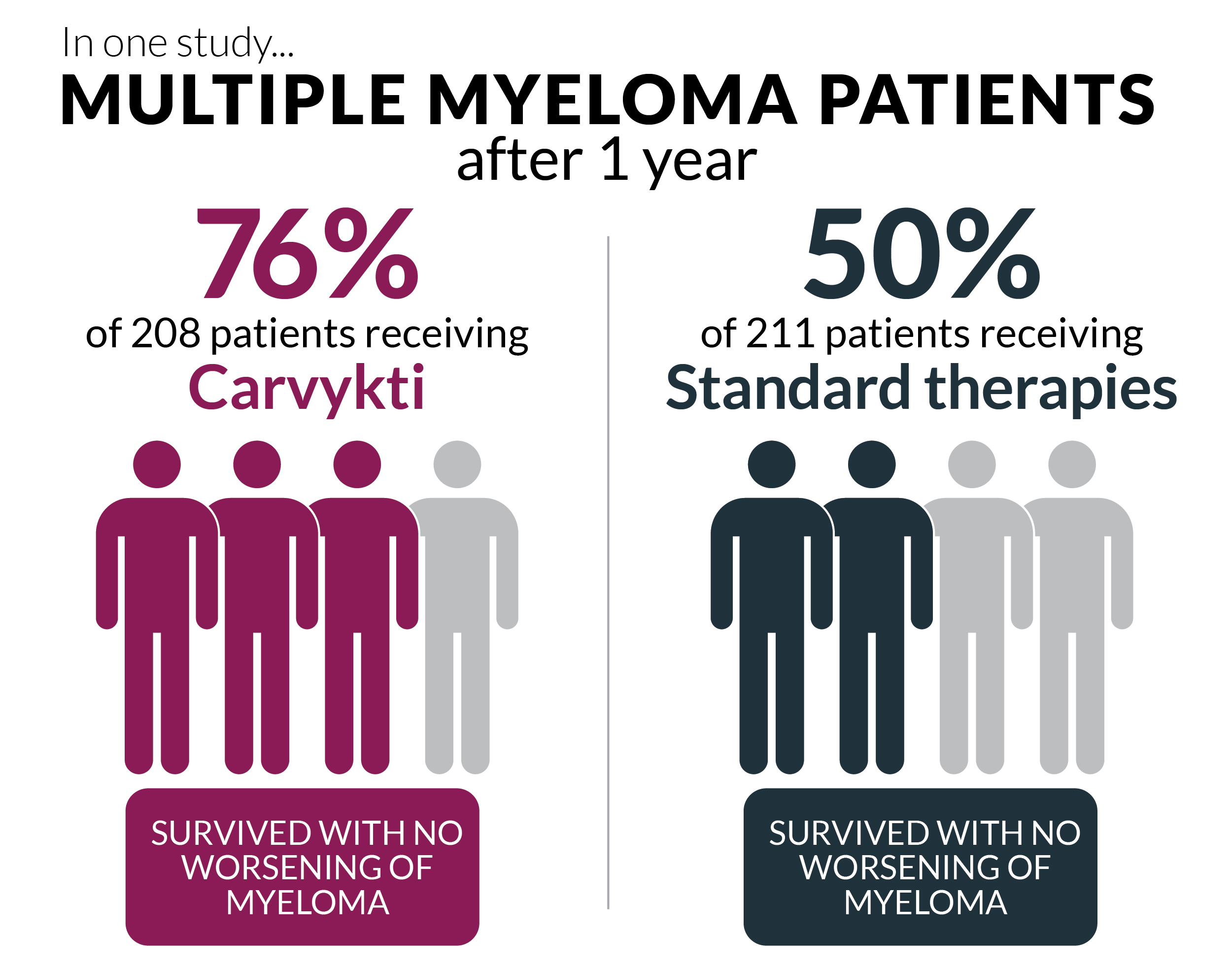
Carvykti is a type of treatment that uses the T cells of your immune system to fight cancer.
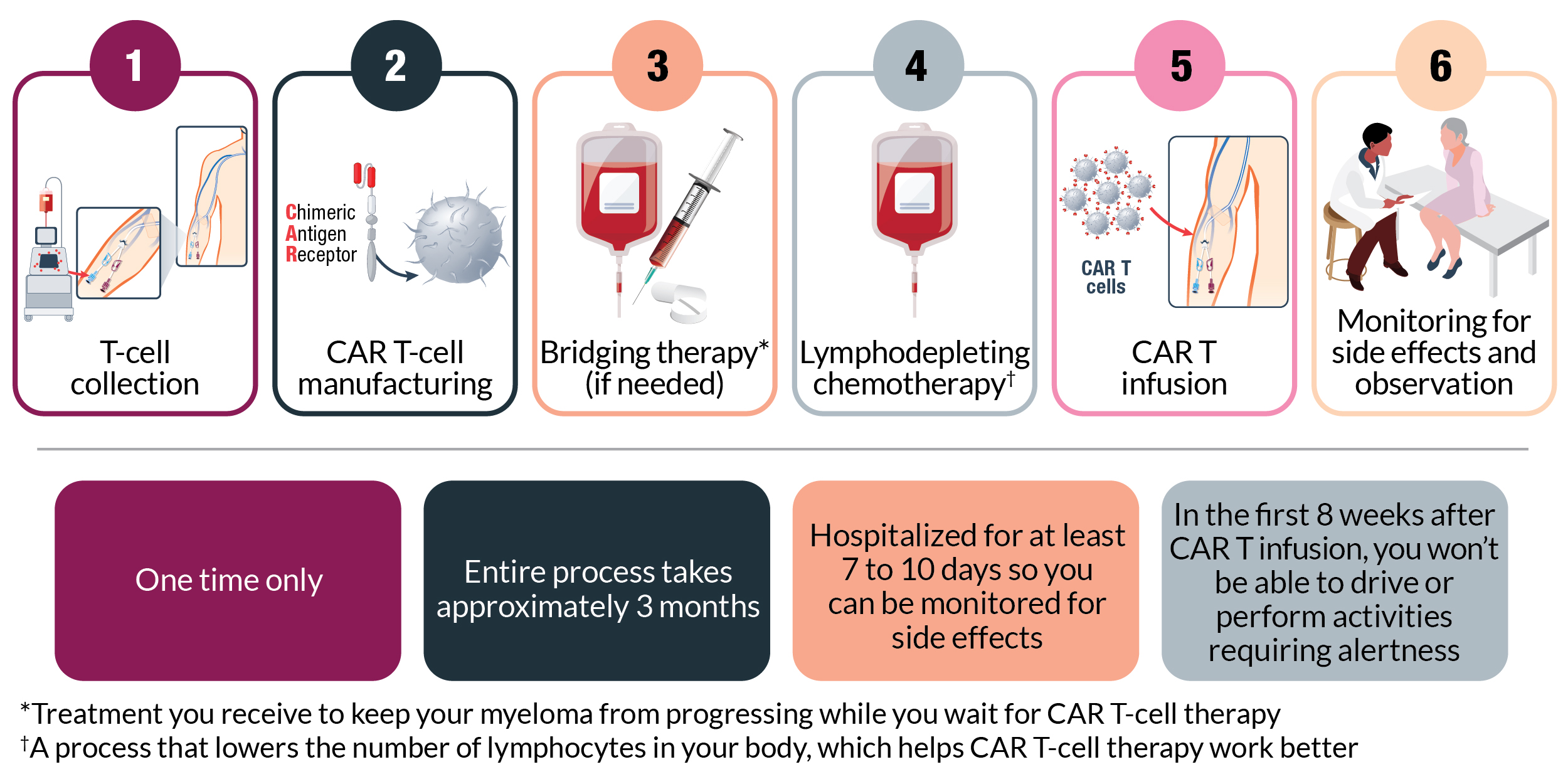
As with other powerful cancer therapies, Carvykti comes with some risks. The most serious potential side effects are cytokine release syndrome and neurologic symptoms, which can be managed by careful monitoring, supportive care, and medication if needed.
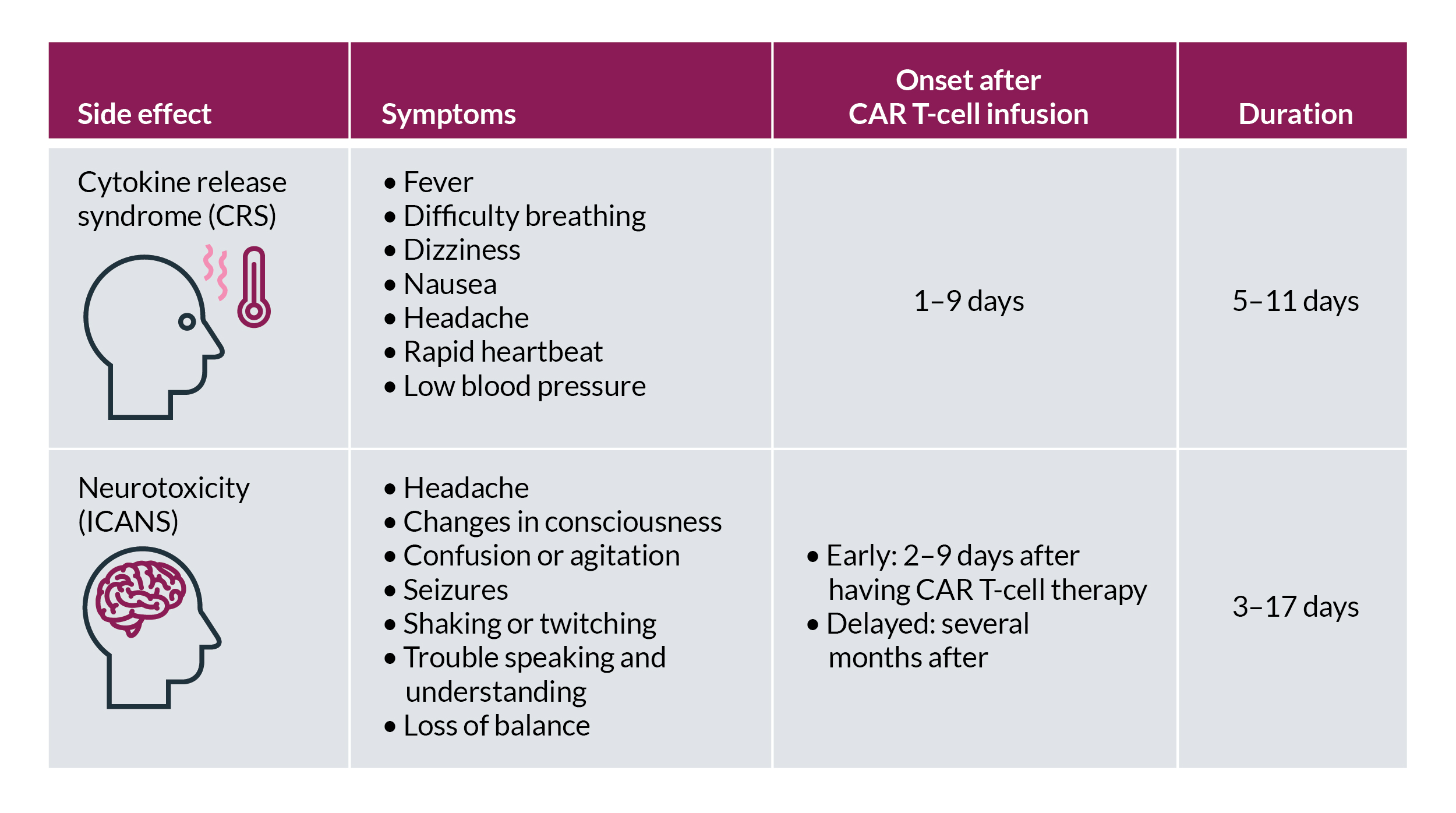
Other common side effects include low blood counts, fatigue, infections, nausea, and muscle or joint pain. You’ll be monitored regularly for side effects, and your care team will provide support to manage them if they develop. Some side effects, particularly neurologic symptoms, can occur well after you receive Carvykti. For this reason, your care team will continue to monitor you for months. Your caregiver should also monitor side effects and communicate any changes to your care team.
Although Carvykti is not for all myeloma patients, it is an exciting step forward for the treatment of relapsed or refractory myeloma. With this updated indication, Carvykti may be available even earlier in the myeloma treatment journey. If you or a loved one has myeloma, talk to your doctor to see if Carvykti might be a good treatment option.
Interested in learning more about Carvykti? Talk to an MMRF patient navigator at the Patient Navigation Center at 1.888.841.MMRF (Monday through Friday, 9:00 am to 7:00 pm ET) or email [email protected].
Additional Resources
Johnson & Johnson. CARVYKTI (ciltacabtagene autoleucel) Patient Support Program.
MMRF. Fast Facts in Myeloma. A Guide to CAR T-Cell Therapy in Myeloma.
MMRF. MMRF Patient Toolkit. Immunotherapy.
MMRF. MMRF Patient Toolkit. Multiple Myeloma Treatment Overview.

