
Every multiple myeloma patient has different disease characteristics. To better understand your prognosis and find the treatment options that are best for YOU, your healthcare team will administer a number of different tests—diagnostic, imaging, and, most importantly, genomic (to determine the genetic makeup of your myeloma).
Diagnostic testing for multiple myeloma and its precursor conditions includes a range of blood tests, urine tests, and bone or bone marrow tests. Undergoing the appropriate multiple myeloma tests is important, as the results help your doctor better determine treatment options and assess your prognosis. Many of these tests are also used to monitor treatment effectiveness.
If you have or are suspected of having myeloma or one of its precursor conditions, you may find that giving blood samples is a common occurrence when you meet with your health care team. Though the process of giving blood samples can be an inconvenience, there are a number of tests that can be run on these samples that are extremely useful to selecting, guiding, and monitoring your treatment.
For a summary explaining the different blood tests used in myeloma diagnosis, please watch our Learn Your Labs HIT Video.
A common characteristic of multiple myeloma is low blood cell counts. Blood cell counts become low in myeloma because the myeloma cells in the bone marrow crowd out and cut off the production of normal blood cells. For more information on the effects of multiple myeloma, see Understanding Multiple Myeloma.
Complete blood count (CBC) tests count the number of different cells and components in your blood—red blood cells, white blood cells, and platelets. By comparing your CBC test results with the ranges for a typical healthy person, your healthcare team can determine the extent to which multiple myeloma is interfering with the normal production of blood cells.
A blood chemistry profile measures the levels of several materials in the blood; these include albumin, calcium, blood urea nitrogen (BUN), creatinine, and lactate dehydrogenase (LDH). The results are used to assess how well the kidneys and liver are functioning, indicate whether myeloma-related bone loss has occurred, and determine the extent of multiple myeloma that is present.
LDH is an important enzyme that is found in all the cells in our bodies. When levels of LDH in the blood are unusually high, it could indicate that myeloma cells are rapidly dividing—a sign of aggressive disease.
The LDH value—along with other blood test results generated at diagnosis—helps your doctor stage myeloma and determine your prognosis.
Beta 2-microglobulin (ß2M) is a protein found on the surface of most cells. When ß2M is found in the blood, it indicates the presence of multiple myeloma; higher levels of ß2M in the blood indicate a greater extent of myeloma. ß2M is also an indicator of kidney function. Higher ß2M levels can indicate that a patient has extensive kidney disease, which helps your doctor determine the stage of your myeloma.
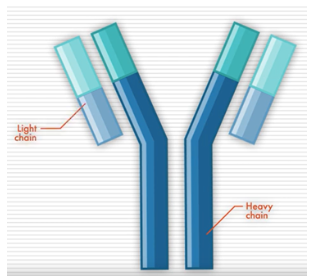
Antibodies, also called immunoglobins (Igs), are proteins produced by cells of the immune system. They help your body recognize something (like a bacteria or virus) as foreign and trigger your immune system to eliminate it. There are five different types of antibodies in our bodies: IgA, IgG, IgM, IgD, and IgE.
Antibodies have a distinct shape, formed by two long proteins (heavy chains) and two shorter proteins (light chains). Determining the levels and types of these antibodies, which are overproduced by myeloma cells, can be helpful in detecting myeloma.
A test called serum protein electrophoresis (SPEP) uses an electrical charge to separate proteins in a blood sample. It is used to detect the presence and levels of various proteins, including M protein (the abnormal antibody protein produced by myeloma cells), in the blood.
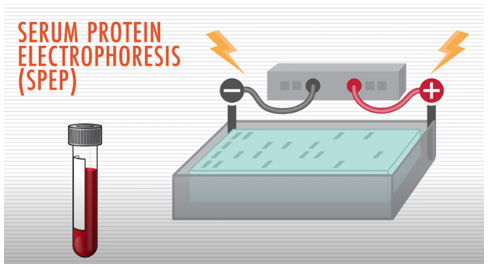
Lower levels of M protein, though not indicative of multiple myeloma, may indicate MGUS.
A high M protein level—referred to as an M spike—indicates that a patient has SMM or multiple myeloma. With multiple myeloma treatment, the M protein levels usually fall. In a patient that has already received treatment, an increase in the M protein level can be a sign that they are relapsing, meaning their myeloma is coming back. For this reason, M protein levels in the blood are useful in monitoring how effective your treatment has been.
Immunofixation electrophoresis (also called immunoelectrophoresis) is another test that uses an electrical current to separate proteins in the blood. This test identifies the type of abnormal antibodies present in the blood (for example, IgM, IgG, or IgA) and helps classify the disease.
The serum free light chain assay is a test that measures antibody light chains, which are proteins produced by plasma cells and make up part of antibodies. Antibody light chains (also called Bence Jones proteins) are classified as either kappa or lambda.
Normally, a person without myeloma has roughly the same number of kappa and lambda light chains, so a serum free light chain assay would show a ratio of one.
Because myeloma cells mainly produce only one type of light chain, assay results that show a higher level of either kappa or lambda light chains can be a sign that a person has myeloma or a related disease.
During multiple myeloma treatment, the level of the light chain made by the myeloma cells decreases, and the ratio of kappa and lambda light chains should return to normal, so the serum free light chain assay is also a good way to monitor how effective treatment is.
![]()
There are also several tests that can be performed on urine samples that are useful in myeloma management.
For a summary explaining the different blood tests used in myeloma diagnosis, please watch our Learn Your Labs HIT Video.
Urinalysis refers to any of several tests used to assess kidney function. These tests include chemical analyses (measuring levels of blood, glucose, protein, and other substances that might be present in the urine) and sometimes visual examination of the urine through a microscope. Abnormal findings may suggest kidney damage due to multiple myeloma.
Protein levels are measured in urine samples collected over a 24-hour period. This test includes measuring levels of antibody light chains (Bence Jones proteins) that, if present, may indicate multiple myeloma. Higher levels indicate more extensive disease.
Urine-protein electrophoresis determines the levels of specific proteins, including M protein and antibody light chains, in the urine. The presence of these proteins indicates multiple myeloma.
In a bone marrow biopsy, your doctor will remove a small piece of bone that contains marrow—the spongy tissue that is found inside bones. For a bone marrow aspiration, your doctor will remove a small amount of liquid bone marrow, which contains marrow cells. Both of these samples are usually taken from the pelvic (hip) bone using a large needle.
These tests are important for several reasons. First, they can be used to determine the number and percentage of normal plasma cells and myeloma cells in the bone marrow. A level of myeloma cells in the bone marrow that exceeds 10% confirms a diagnosis of multiple myeloma; a higher percentage indicates more extensive disease.
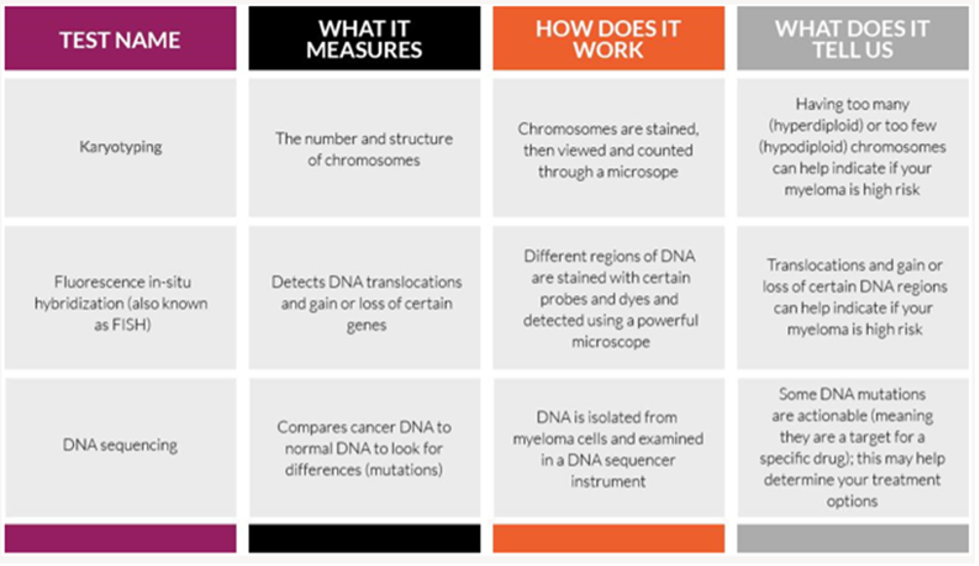
Second, myeloma cells that are collected from your bone marrow can be used to perform cytogenetic testing (including FISH, karyotyping, and genomic sequencing), which is vital in determining any genomic mutations and may also be useful in assessing risk stratification for determining what your treatment plan should be.
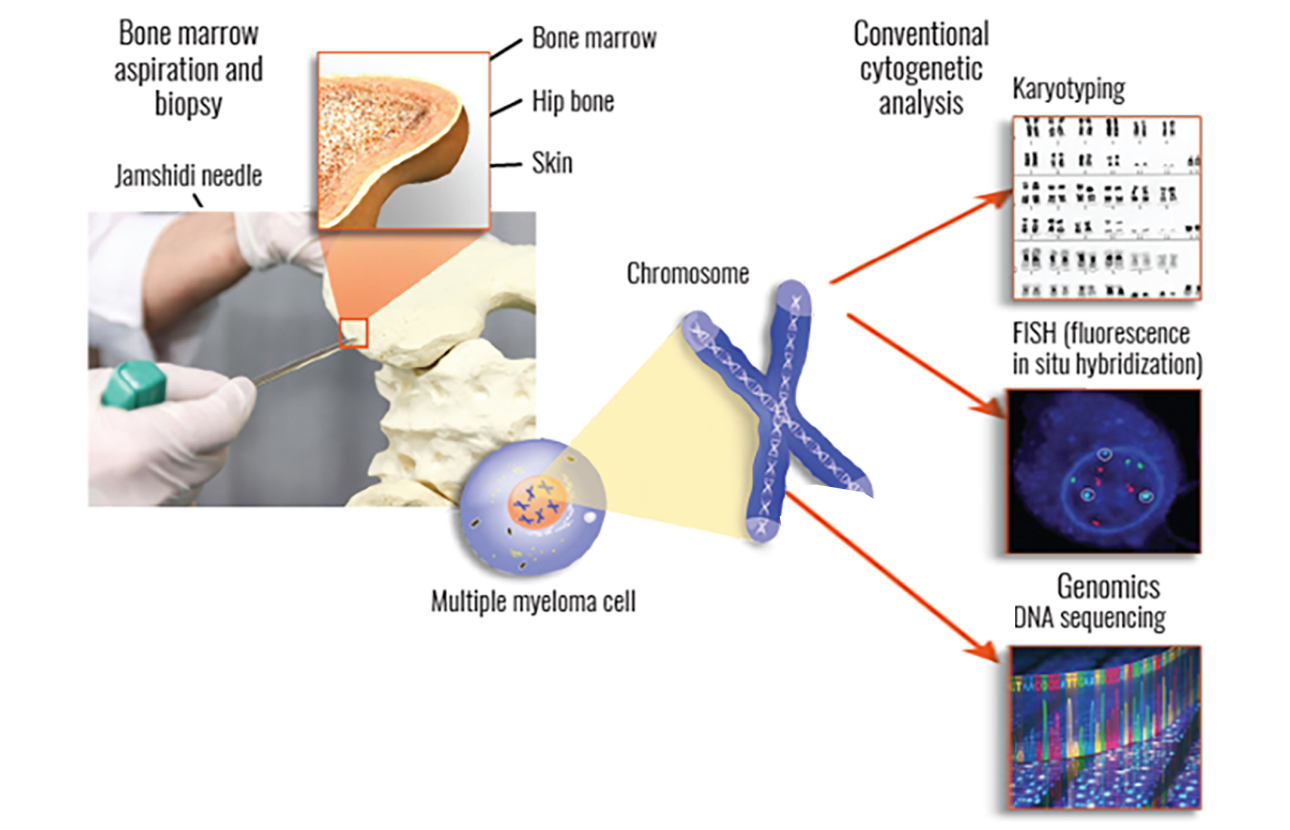
Bone marrow biopsy testing should be done at the time of diagnosis and might be repeated when the myeloma relapses.
One of the main tests performed on bone marrow samples is fluorescence in situ hybridization, or FISH. This test provides information about chromosomes specifically.
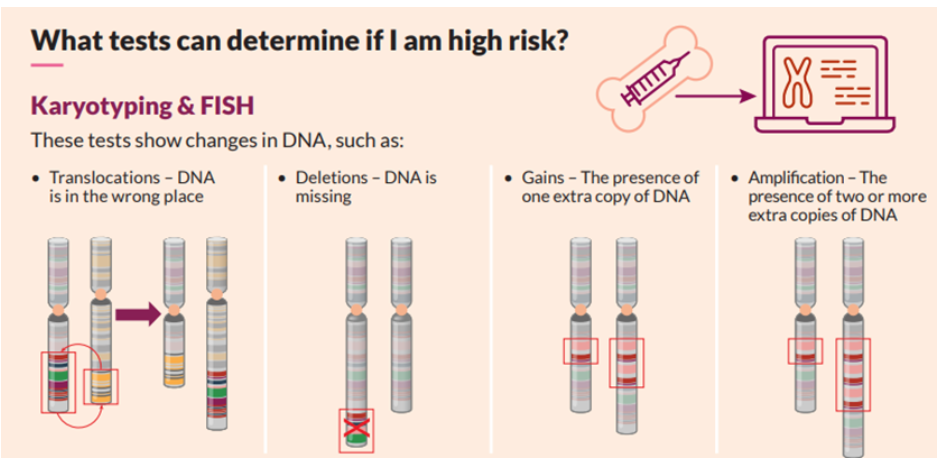
Certain changes in the chromosomes are associated with the development of myeloma. The FISH test only looks at abnormalities in the myeloma cells.
Another test that may be done on bone marrow samples is karyotyping, which involves looking at the size, shape, and number of chromosomes in a sample. Some of the information provided by karyotyping overlaps information provided by FISH, like hyperploidy, deletions, or translocations. FISH may be more sensitive in detecting some aberrations but is not as widely available.
It is strongly recommended that multiple myeloma patients undergo genomic sequencing when possible. Genome sequencing can provide valuable information about your prognosis, your treatment options, and how your myeloma is changing in response to treatment.
Multiple myeloma genome sequencing is the process of examining the DNA of individual myeloma cells. The DNA in the myeloma cells is made up of the same molecules as the DNA in normal cells, but it has been altered. When these genetic alterations (called mutations) develop, the proteins produced in those cells can no longer do the job they’re supposed to do and instead start doing what the mutated DNA is instructing them to do (for example, causing myeloma cells to continue to grow and multiply out of control).
Sequencing DNA allows a doctor to understand how your tumor is working—how it grows, how it is trying to avoid detection by the immune system, and even how it might respond to specific therapies.
From a genetic perspective, not all multiple myeloma is the same. Genome sequencing can give you and your care team information about your prognosis, your treatment options, and how your myeloma is changing in response to treatment.
Genome tests table
Along with blood and urine tests, imaging tests can provide valuable information about the status of your myeloma. Imaging tests conducted on bone help the healthcare team diagnose multiple myeloma and are used to monitor the disease during treatment.
Bone imaging studies
As multiple myeloma gets worse, it causes small holes (lytic lesions) to develop in the bones. A number of imaging tests are used to locate and measure lytic lesions, including bone (skeletal) survey, x-ray, magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET). These tests assess changes in the bone structure and determine the number and size of tumors in the bone. Higher levels of bone changes suggest the presence of multiple myeloma. Some of these tests can also detect multiple myeloma that is outside the bone marrow (extramedullary).