Several presentations at the 2024 American Society of Hematology (ASH) Annual Meeting relayed new and updated study data in multiple myeloma (MM), along with significant advancements in the treatment of
Ongoing research of SMM treatment is focused on early intervention and observation with monitoring, identification of patients at high risk of progression, and novel treatment approaches. Two studies addressed these issues.
3-year PFS of 94% vs 40%. MRD negativity after 4 cycles was not significantly better with KRd (37% vs 14%; OR=0.27, 95% CI 0.06–1.12; P=0.07).
*MAYO criteria: bone marrow plasma cells ≥10%, serum M protein ≥3 g/dL, and serum free light-chain ratio <0.125 or >8; PETHEMA criteria: presence of ≥95% abnormal plasma cells (presence or absence of CD38, CD56, CD19, and/or CD45), and immunoparesis, defined as a reduction (below the lower normal limit) in the levels of 1 or 2 of the uninvolved immunoglobulins.
Two studies highlighted important aspects of prognostic indicators: one addressed functional high-risk disease status and the other assessed the value of routine 24-hour urine assessments.
Functional high-risk status could be a useful tool for identifying patients with poor prognosis, according to one analysis. Functional high-risk MM, characterized by disease progression or death within a year of initial treatment, is a poor prognostic subset regardless of high-risk cytogenetic features at diagnosis. Of the 228 functional high-risk patients identified from the Multiple Myeloma Research Foundation (MMRF) CoMMpass study, 165 were categorized as high risk and 63 as standard-risk (ie, patients with or without high-risk features [t(4;14), t(14;16), t(14;20), 1q amplification (amp1q), 17p deletion (del17p), TP53 mutations, or ISS-stage III disease]). The median overall survival (OS) was 13.2 months for the standard-risk group and 11.6 months for the high-risk group (P=0.29). Primary refractory disease was prevalent in both groups, with OS significantly shorter for patients with high-risk vs standard-risk disease (5.0 vs 7.8 months; P=0.048).
Despite differences in second-line treatment patterns (standard-risk patients received more triplet regimens; high-risk patients relied on doublets), response rates were similar, with median OS for relapsed patients 27.1 months vs 20.2 months (P=0.22 standard- vs high-risk). Overall, functional high-risk patients had an OS <24 months regardless of the presence of high-risk features at diagnosis.
This study confirms that many high-risk disease features do not present with other known high-risk features at diagnosis. More research into reliably identifying patients with high-risk features is needed.
A secondary analysis of the BMT CTN 0702 (STaMINA) trial evaluating whether 24-hour urine testing adds value to MM response assessments based on International Myeloma Working Group (IMWG) criteria found that removing 24-hour urine testing requirements from response criteria resulted in changes to <1% of patient responses, with no impact on PFS prediction at any response depth. Median PFS for traditional and urine-free criteria was identical across response categories, including 63.0 months for complete response (CR) and 49.6 months for very good partial response (VGPR). Urine-free IMWG criteria remained highly prognostic for PFS (P=0.006; HR=1.05–1.36).
The authors concluded that urine-free criteria “reduce time, toxicity, and discomfort for patients, particularly those with limited dexterity,” though they also noted that these assessments remain critical in specific cases, such as AL amyloidosis or urine-only measurable disease.
Several studies highlighted the evolution of induction therapy for improving survival and quality of life for patients with NDMM. Current standard-of-care (SOC) regimens, such as quadruplet therapy incorporating daratumumab or isatuximab with bortezomib-lenalidomide-dexamethasone (VRd), continue to demonstrate superior MRD negativity and PFS regardless of autologous stem cell transplant (ASCT) eligibility, making these regimens an appropriate option for a majority of patients. Data on additional investigational therapies, including teclistamab- and belantamab-based regimens, were also presented.
Notable findings regarding investigational quadruplets in NDMM were reported from two studies.
The phase 2 MajesTEC-5 study evaluated the use of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in combination with daratumumab-lenalidomide-dexamethasone (DRd) or D-VRd as induction therapy for patients with TE NDMM. Out of 49 patients, all assessable patients (n=35) achieved MRD negativity (NGF, 10⁻⁵) after completing 3 treatment cycles, with sustained MRD negativity in those completing 6 cycles. Despite a high rate of cytokine release syndrome (CRS) events (65.3%, all grade 1/2), no cases of neurotoxicity or treatment discontinuation due to AEs were reported. The regimens demonstrated robust clinical efficacy with a manageable safety profile, suggesting their potential for improving long-term outcomes in this setting.
Promising results were reported from the phase 1 DREAMM-9 trial of belantamab mafodotin (belamaf) combined with VRd in TI NDMM patients. Across 8 dosing cohorts involving 108 patients, ORR was high, ranging from 71% to 100%, with the CR rate reaching 92% in certain cohorts. MRD negativity (NGS, 10-5) was most notable in higher-dose groups, achieving rates up to 75% in patients with CRs. Ocular events were the most common AE, with grade 3+ keratopathy and visual acuity scale AEs affecting up to 92% of patients in higher-dose groups, though longer dosing intervals reduced the frequency and delayed the onset of these events.
Also reported at ASH 2024 were findings from two studies on the use of maintenance therapy in MM, including duration of maintenance therapy, role of combination therapies vs single-agent maintenance, MRD status for informing therapy decisions, and the role of novel agents.
A prospective study evaluated whether discontinuing lenalidomide maintenance therapy after 3 years of sustained MRD negativity (NGF) might be feasible and safe in NDMM patients following ASCT. MRD status was assessed in patients who had achieved stringent CR and then at 6, 12, 24, and 36 months after the initiation of lenalidomide maintenance. Patients who had at ≥3 consecutive MRD-negative results and had received at least 36 months of maintenance discontinued lenalidomide maintenance only if they had also achieved imaging MRD negativity (via PET/CT scan). MRD was performed every 6 months thereafter. At 3 years, 26.3% of patients (N=194) achieved sustained MRD negativity in bone marrow and imaging and discontinued maintenance. Over a median follow-up of 32 months after discontinuation, 96% of these patients remained MRD negative at 6 months, with a gradual decline to 86% at 3 years. Median PFS for this cohort was 74 months (95% CI, 38–104 months).
According to the authors, “sustained MRD negativity after ASCT and completion of 3 years of lenalidomide maintenance may guide the safe discontinuation of maintenance” They also noted, however, that further validation in randomized trials is needed.
Several studies demonstrated that sustained MRD negativity with maintenance therapy is associated with improved outcomes. Whether TE or TI, patients showing deeper responses to therapy had prolonged PFS and OS, regardless of therapeutic approach.
Results from another study suggested that, in NDMM patients treated with quadruplet therapy and ASCT, MRD progression (defined as a ≥1-log10 increase in MRD burden) was associated with a median time of 10.1 months to IMWG-defined progression.
The authors concluded that MRD progression challenges reliance on paraprotein-based progression criteria, suggesting the need for earlier intervention and therapies with novel mechanisms of action.
An analysis of the GEM2017FIT trial demonstrated that peripheral residual disease (PRD) assessed by mass spectrometry and MRD assessed by NGF have significant prognostic value for PFS in older TI NDMM patients.
According to the researchers, these findings support the integration of PRD evaluation into routine clinical practice to complement MRD, improving risk stratification and informing treatment strategies for older MM patients. Taken together, these findings highlight the role of PRD and MRD negativity as a robust prognostic marker and treatment goal in NDMM.
Several studies suggested that pretreatment biomarkers and treatment sequencing are important considerations for optimizing therapeutic responses and minimizing toxicity in relapsed MM.
MM patients had significantly shorter PFS with BCMA-directed CAR T-cell therapy if they had previously received an ASCT, according to one report. A study involving 104 patients indicated that although use of HDM/ASCT did not influence the CR rate (P=0.42), patients treated with prior HDM/ASCT experienced a shorter median PFS with CAR T therapy (9.5 vs 21 months; P=0.01). Multivariate analysis confirmed this association (HR=2.17, 95% CI, 1.25–3.74; P=0.006), independent of other factors such as high-risk cytogenetics or prior treatments. Notably, the timing between HDM/ASCT and CAR T did not influence PFS, and prior HDM/ASCT had no effect on OS, CRS, or ICANS. The authors conclude that these findings may help inform treatment sequencing in MM to optimize outcomes.
Factors influencing toxicity and durable responses were identified in a comprehensive analysis of pretreatment biomarkers in 108 patients receiving idecabtagene vicleucel (ide-cel) for RRMM. High inflammatory markers (eg, ferritin, IL-6, IL-15; P<0.05) at baseline and elevated plasma cell burden (≥50%; P=0.02) were associated with a higher risk of ICANS, whereas higher cell doses correlated with increased CRS severity (median dose 440 vs 411 × 10⁶; P=0.01). Durable responses (defined as PFS at ≥9 months) were linked to favorable bone marrow profiles, including higher CD4:CD8 ratios and increased cytotoxic natural killer and central memory CD8+ T cells. Conversely, nondurable responses were associated with elevated inflammatory markers, prior BCMA exposure, and high levels of myeloid-derived suppressor cells (P=0.0025). Given these findings, pretreatment evaluation may help optimize patient selection and improve outcomes with ide-cel therapy.
Findings suggest that response to teclistamab may depend on the timing and type of prior BCMA-directed therapy, offering insights into treatment sequencing in RRMM. In a multicenter study evaluating teclistamab in RRMM, prior exposure to BCMA-directed therapy was associated with a lower ORR (51.4% vs 61.5%; P=0.012) and shorter PFS (median 4.6 vs 8.2 months; P=0.017) than was seen in patients not previously treated with BCMA-directed therapies. Although prior BCMA-directed therapy was not independently predictive of PFS (HR=1.25, 95% CI, 0.95–1.64; P=0.1), waiting >8.7 months between BCMA therapies was linked to superior PFS (8.1 vs 2.5 months; P=0.001). Toxicity profiles were comparable between groups, but grade 3 thrombocytopenia occurred more frequently in the BCMA-exposed cohort (10.7% vs 6.6%; P=0.08).
Several studies on the treatment of early RRMM were presented. Belantamab mafodotin–based regimens demonstrated superior PFS to and deeper responses than daratumumab-based combinations, offering a potential new option for first-relapse treatment.
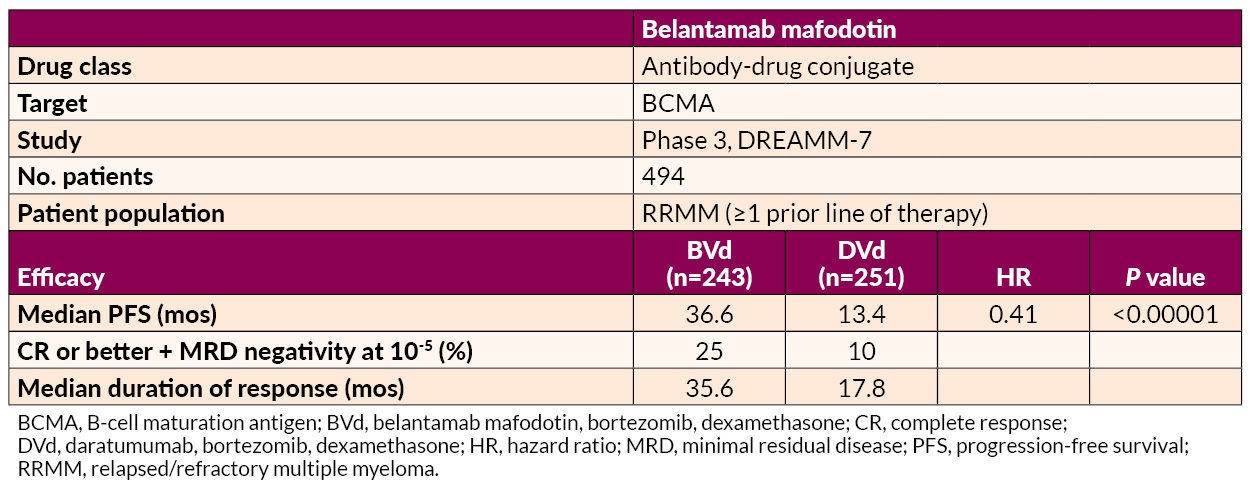
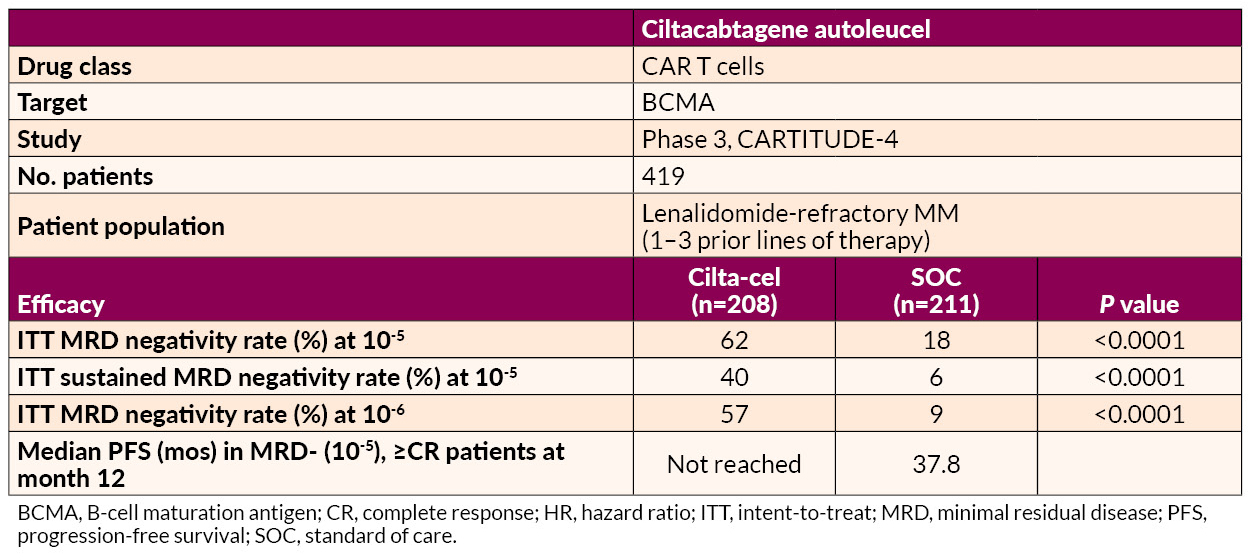
Ciltacabtagene autoleucel (cilta-cel) delivered significant benefits in MRD negativity and prolonged PFS relative to SOC in lenalidomide-refractory patients.
Mezigdomide in combination with bortezomib or carfilzomib achieved high response rates (up to 85.7%) and durable responses (median PFS up to 17.5 months) across dose-expansion cohorts. Mezigdomide in other novel combinations, such as with agents targeting key oncogenic pathways (eg, MEK and BET inhibitors), displayed promising efficacy with manageable toxicity profiles.
Elranatamab combined with carfilzomib and dexamethasone demonstrated promising efficacy with a 100% ORR and manageable safety signals, including no dose-limiting toxicities in early-phase trials.
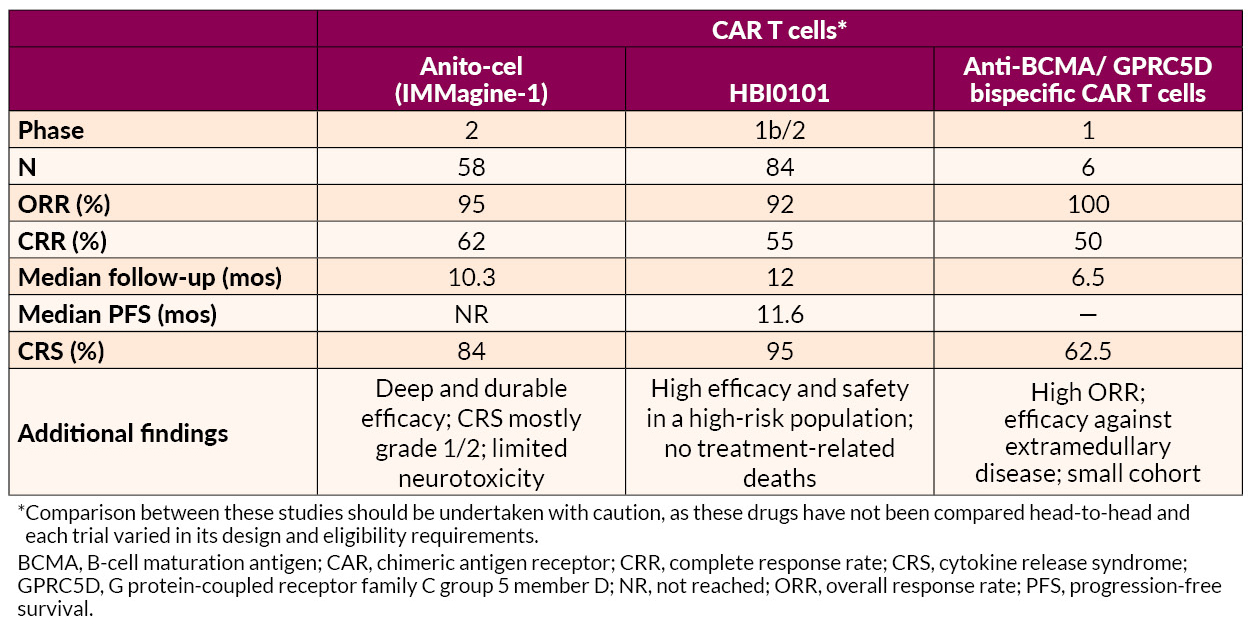
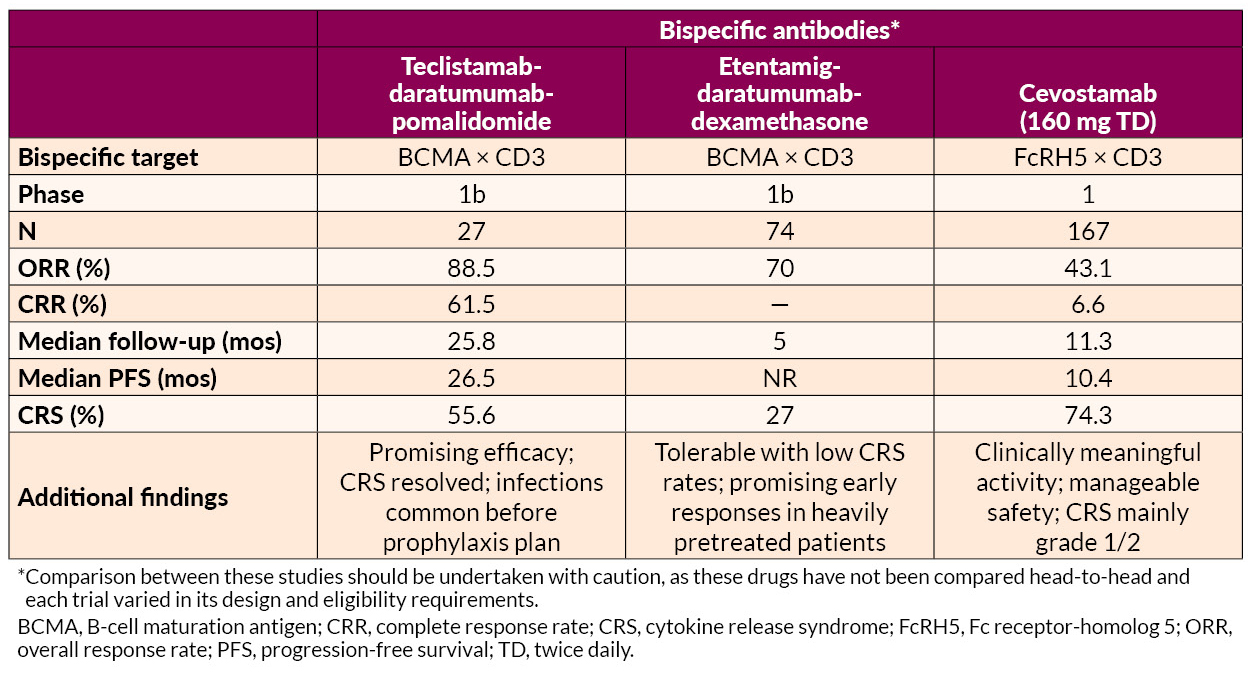
Several novel approaches to late treatment of RRMM with CAR T cells, including BCMA-targeted anitocabtagene autoleucel (anito-cel) and HBI0101 and bispecific antibodies, also showed promise.
AL amyloidosis is a plasma cell disorder and occurs in 10% to 15% of MM patients. Studies presented on AL amyloidosis showed benefits for novel drug combinations and CAR T-cell therapy. In addition, one analysis suggested that further development of therapies targeting deposited amyloid fibrils may be useful for addressing cardiac dysfunction associated with AL amyloidosis.
The median MOD-PFS was not reached for daratumumab-VCd vs 30.2 months for VCd. The 5-year survival rates were 76.1% with daratumumab-VCd vs 64.7% with VCd. Cardiac and renal responses were approximately doubled in the daratumumab-VCd group.
Treatment responses were observed early on, with a median time to initial hematologic response of 1 week and ≥VGPR within 4 weeks, including in patients with poor initial responses.
Another study found that the novel anti-BCMA-targeted CAR T-cell therapy HBI0101 demonstrated high efficacy and acceptable safety in 16 heavily pretreated patients with relapsed/refractory AL amyloidosis.
The authors noted that earlier intervention before advanced cardiac disease may optimize outcomes, but HBI0101 shows potential to improve organ function and survival in this difficult-to-treat population.
Further development of therapies targeting deposited amyloid fibrils may be needed to address cardiac dysfunction in AL amyloidosis, according to one report. A single-site retrospective study of 43 patients highlighted the fact that although current systemic AL amyloidosis treatments, including daratumumab- and bortezomib-based regimens, effectively reduce toxic amyloid light chain production, they show limited impact on improving cardiac function. The study showed that 35% of patients experienced clinically significant improvement in cardiac dysfunction, as measured by global longitudinal strain (GLS), but only 10% transitioned from reduced to normal GLS. Most patients (79%) had reduced GLS at baseline, with limited meaningful changes observed across follow-up periods.
—
Jointly provided by the MMRF and RedMedEd.
Support for this activity has been provided through sponsorships from Alexion Pharmaceuticals, Inc.; Pfizer Inc.; and Sanofi US and by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC.
Welcome to our final recap of the latest research on myeloma treatments presented at the American Society of Hematology (ASH) Annual Meeting in San Diego. Highlights from the final day included:
Let’s jump into the latest findings!
Researchers from Greece demonstrated that treatment with Darzalex can significantly delay the progression from smoldering multiple myeloma (SMM) to active disease in high-risk patients. Data from this abstract, simultaneously published in the New England Journal of Medicine (Dimopoulos et al.), showed that patients receiving Darzalex had a 51% lower risk of progression (that is the time until myeloma is detected) compared to those under active monitoring, the current standard of care after a median of more than 5 years of follow-up. The most common serious side effect in both arms was hypertension, affecting approximately 5%. Approximately 5% of patients in the Darzalex arm discontinued treatment.
High-risk SMM is an asymptomatic precursor to multiple myeloma, and early treatment strategies may improve outcomes for patients with a high risk of progression to active disease. The researchers emphasized that findings from this phase 3 trial specifically apply to patients with high-risk SMM. Future research will explore whether Darzalex alone or in combination with other medications could help prevent disease progression in patients with high-risk SMM.
Two abstracts showed that Sarclisa in combination with the current standard of care demonstrated significant treatment benefits in patients with newly diagnosed multiple myeloma (NDMM). Sarclisa is an anti-CD38 antibody in the same class as Darzalex and is administered intravenously.
The first abstract showed that Sarclisa in combination with Revlimid (lenalidomide), Velcade (bortezomib), and dexamethasone (Isa-VRd) induction therapy resulted in a 30% reduction in the risk of disease progression in transplant-eligible NDMM patients compared to VRd. Half of patients treated with Isa-VRd achieved minimal residual disease (MRD) negativity, indicating no detectable cancer cells, after 18 weeks of treatment. Side effects such as infections, diarrhea, and peripheral neuropathy were consistent with previous clinical trial findings of Isa-VRd.
In the next abstract, Dr. Orlowski and colleagues from MD Anderson Cancer Center reported that Isa-VRd induction therapy followed by maintenance with Isa-Rd demonstrated higher and sustained MRD negativity rates compared with the current standard of care in NDMM patients not eligible for a transplant. This analysis of the phase 3 study included 446 patients. Side effects of Sarclisa observed in this study were consistent with prior studies of Sarclisa and VRd.
Researchers conclude that Sarclisa-based quadruplets may represent an alternative new standard of care for patients with NDMM.
For patients with relapsed or refractory multiple myeloma considering treatment with Abecma (idecabtagene vicleucel), a CAR T-cell therapy, understanding factors that influence the effectiveness and side effects is important. Currently it is available for patients who have had two or more prior lines of therapy.
In a study of 108 patients, Dr. Hansen and researchers from the H. Lee Moffitt Cancer Center found that patients with high disease burden, such as advanced disease stage, and the presence of biomarkers of inflammation like ferritin and C-reactive protein before starting Abecma treatment were more likely to develop side effects such as neurotoxicity (ICANS). Patients who had extramedullary disease (ie, the presence of myeloma cells outside of the bone marrow) and those who had received prior treatment with a BCMA-targeting agent were less likely to have durable responses.
These findings highlight the importance of pre-treatment factors in shaping outcomes with ide-cel therapy and may help guide personalized approaches to optimize benefits while managing risks.
In the next abstract, researchers in the United Kingdom reported that treatment with Carvykti led to significantly higher rates of MRD-negativity in patients with RRMM who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), compared to standard therapies of Pomalyst (pomalidomide), Velcade, and dexamethasone (PVd) or Darzalex, Pomalyst, and dexamethasone (DPd). Carvykti is currently available for patients who have had one or more prior lines of therapy. These findings further support the use of Carvykti patients with Revlimid-refractory MM.
Dr. Dima and researchers at the Cleveland Clinic Taussig Cancer Institute looked at how effective Tecvayli, a BCMA-targeting bispecific antibody, is for patients with relapsed/refractory multiple myeloma (RRMM) who previously received other BCMA-directed therapies like CAR T-cell therapy or antibody-drug conjugates (ADC). In this abstract, researchers found that while Tecvayli is effective for these patients, outcomes were generally better for those who hadn’t received prior BCMA treatments. Currently, it is available for patients who have had four or more lines of therapy.
Patients previously treated with BCMA-directed therapy had slightly lower response rates to and shorter median progression-free survival (PFS, time before the disease worsened) compared to for those without prior BCMA-directed therapies. The study also highlighted the importance of timing between BCMA-directed therapies. Patients who waited more than 9 months after their last BCMA-directed therapy before starting Tecvayli had better outcomes. Side effects like CRS and neurotoxicity (ICANS) were similar regardless of prior BCMA therapy; however, patients with prior BCMA-directed therapy were more likely to experience low platelet counts early in treatment. These findings suggest that Tecvayli is a valuable option for patients previously treated with BCMA therapies, and patients and caregivers should discuss the timing of starting Tecvayli with their healthcare team.
Dr. Tomasson and colleagues from the University of Iowa reported that Elrexfio, a bispecific antibody targeting BCMA, in combination with Kyprolis (carfilzomib) and dexamethasone shows promise for treating patients with RRMM. Currently, Elrexfio is available for patients who have had four or more lines of therapy.
In this abstract, researchers data from an early-phase study involving 12 patients, all of whom had received at least one or more prior lines of therapy. The most common side effects included fatigue, mild cytokine release syndrome (CRS), and low blood counts. Further studies ongoing will evaluate the benefits of Elrexfio combined with Kryprolis and dexamethasone in a larger population.
Researchers from Brazil reported that belantamab mafodotin combined with Velcade and dexamethasone (BVd) shows significant overall survival benefit, reducing the risk of death by 42% in multiple myeloma at or after first relapse compared to Darzalex with Velcade and dexamethasone (DVd). In this abstract, data was analyzed from 494 patients, of which 51% of patients had received 1 previous line of therapy, 52% had received prior Revlimid, while 34% of patients no longer responded to Revlimid; 28% had high-risk chromosomal abnormalities. The researchers note that additional studies will shed light on if BVd may be a potential new standard of care in MM at first relapse or later.
Cevostamab is an investigational bispecific antibody that targets FcRH5, a new target on myeloma cells. In this study of 167 heavily pretreated patients (between 2 and 18, with a median of 6), Dr. Richter and researchers at Mount Sinai found that cevostamab demonstrated good response rates. Patients who had not previously received BCMA-targeted therapies responded better than those who had. Almost all patients (96%) were resistant to all three standard treatment classes: proteasome inhibitors, immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies. A majority (57%) of patients had received one or more prior BCMA-targeted therapy. Common side effects included CRS, low white blood cell counts, and fatigue. Further analysis will evaluate the use of cevostamab in larger trials.
CELMoDs are a new class of oral myeloma drugs that work like immunomodulators such as Revlimid and Pomalyst. They also stimulate the immune system and kill myeloma cells directly, even for myeloma that has become resistant to certain treatment. Researchers from Canada presented their findings from an early phase trial evaluating mezigdomide, an oral CELMoD, in combination with dexamethasone and either Velcade (Mezi-Vd) or Kyprolis (Mezi-Kd). In this study, 77 patients who have had one or more prior lines of therapy received Mezi-Vd and 27 patients received Mezi-Kd. The authors reported that Mezi-Vd and Mezi-Kd demonstrated high response rates and side effects such as low white blood cell counts, infections, and low platelet counts.
Researchers from Israel reported data from an early phase clinical study of HBI0101, an anti-BCMA CAR T-cell therapy. Their study included 84 patients, most of whom were triple class refractory, showed high response rates and MRD negativity rates in patients treated with HBI0101. Common side effects included CRS, low white blood cell, low red blood cell, and low platelet counts.
Dr. Freeman and researchers at the H. Lee Moffitt Cancer Center reported their findings from an early phase trial evaluating anitocabtagene autoleucel, another anti-BCMA CAR T-cell therapy. The trial included 58 patients with RRMM who have received at least three prior lines of treatment. Patients treated with anitocabtagene autoleucel achieved high response rates and MRD negativity rates. Common side effects observed were CRS, low white blood cell, low red blood cell, and low platelet counts.
Further analysis will evaluate the use of these new therapies in larger trials.
We hope you enjoyed our daily recap of the latest advances in myeloma treatment. Keep an eye on themmrf.org for future updates!
Welcome to the second day of our recap of the latest findings on myeloma treatments reported at the American Society of Hematology (ASH) meeting in San Diego.
Highlights from today included:
Let’s take a closer look!
Several abstracts highlighted promising results on the use of Tecvayli (teclistamab) in combination with other drugs for patients with newly diagnosed multiple myeloma (NDMM) and those who relapse after one or more line of treatment. Currently, it is available for patients who have had four or more lines of therapy.
In the first abstract, 49 patients with transplant-eligible NDMM were treated with Tecvayli in combination with Darzalex, Revlimid, and dexamethasone with or without Velcade (Tec-DRd) or (Tec-DVRd) as induction therapy. Researchers from Germany reported that 35 of 36 patients who completed at least 3 treatment cycles achieved minimal residual disease (MRD) negativity, indicating no detectable cancer cells. The most common side effects were infections and cytokine release syndrome (CRS), which presents as flu-like symptoms and is a common side effect of the bispecific antibodies. Further analysis will evaluate these combinations in larger trials.
Revlimid maintenance therapy after autologous stem cell transplantation (ASCT) has been the standard-of-care for transplant-eligible NDMM. However, patients eventually relapse, supporting the need for better strategies for maintenance therapy. In this abstract, researchers from Italy reported data from an early phase trial that Tecvayli with or without Revlimid could be safely given as maintenance therapy for 90 patients with transplant-eligible NDMM. Common side effects included infections, low white blood cell counts, CRS, cough, diarrhea, and fatigue. All patients enrolled in the study who received Tecvayli with or without Revlimid as maintenance therapy achieved MRD negativity. These findings support the ongoing study of Tecvayli alone or in combination with Revlimid as maintenance therapy.
In the next abstract, Dr. D’Souza and colleagues at the Medical College of Wisconsin showed that Tecvayli in combination with Darzalex and Pomalyst may lead to better responses, especially in patients with relapsed or refractory multiple myeloma (RRMM) who had one or more lines of therapy. Common side effects reported in this early phase trial of 27 patients with RRMM were infections, low white blood cell, red blood cell, and low platelet counts. The researchers concluded that, pending the results of a larger study, Tecvayli in combination with Darzalex and Pomalyst may be a new triplet therapy for early relapsed myeloma.
Etentamig (formerly ABBV-383) is a new bispecific antibody that targets B-cell maturation antigen (BCMA) that is being evaluated with monthly dosing from the beginning of treatment, unlike Tecvali and Elrexfio which are administered weekly through 24 weeks after initial step-up.
Dr. Rodriguez and colleagues at Mount Sinai presented results from an early phase trial of ABBV-383 combined with Darzalx and dexamethasone in this abstract. The study, which aimed to identify the best dosage of ABBV-383, included 60 patients who received 3 or more prior lines of therapy including a proteasome inhibitor and an immunomodulatory drug. Of this group 70% had received anti-CD38 mAb therapy. Most common side effects included low platelet, white blood cell, and red blood cell counts, CRS and fatigue. Their findings suggest that ABBV-383 in combination with Darzalx and dexamethasone is tolerable and early response rates were promising. Ongoing trials will offer more information on the potential use of ABBV-383 in RRMM.
In this abstract, Dr. Usmani and colleagues from Memorial Sloan Kettering Cancer Center showed belantamab mafodotin combined with VRd led to good response rates in patients with NDMM who are ineligible for transplant. Vision problems were the most common side effect. These side effects were managed by briefly stopping treatment or reducing the dose of belantamab mafodotin, allowing most patients to continue treatment. With these promising results, future studies will continue to evaluate the potential of belantamab mafodotin in patients with newly diagnosed disease.
In the final abstract, Dr. Foster colleagues at the University of Virginia reported that Darzalex in combination with Revlimid maintenance therapy resulted in better remission rates (MRD-negative) and longer disease control compared to Revlimid alone. The researchers evaluated data from the phase 3 AURIGA study that included 200 transplant-eligible NDMM patients who were MRD-positive after ASCT regardless of age, race, or risk profile. Findings from this trial revealed that maintenance therapy with Darzalex combined with Revlimid prolonged the time to disease progression across all subgroups of patients, including those with high-risk disease. Low white blood cell counts and infections were the most common side effects observed in this phase 3 trial of 200 patients with NDMM.
The researchers conclude that these results further support adding Darzalex to Revlimid maintenance therapy for patients with NDMM following stem cell transplant.
Be sure to come back tomorrow for our final day of updates in the treatment of myeloma at ASH 2024.
Welcome to our 2024 recap of the latest research on myeloma treatments reported at the American Society of Hematology (ASH) meeting that kicked off Saturday in San Diego. Highlights from today included:
Let’s dive in!
Autologous stem cell transplantation (ASCT) followed by Revlimid (lenalidomide) maintenance continues to be the standard of care for eligible patients with newly diagnosed multiple myeloma (NDMM). Currently, patients with myeloma typically stop maintenance therapy when their disease progresses, meaning there is no set timeframe to stop treatment. However, researchers are finding that patients with sustained (durable) minimal residual disease (MRD) negativity (that means that no myeloma cells were detected) may be able to safely stop maintenance therapy.
In this abstract, researchers from Greece found that maintenance therapy could be stopped for certain patients might be able to stop taking Revlimid maintenance therapy if they were consistently MRD-negative for 3 years. The study included 194 patients who received induction with proteasome inhibitor-based regimens (ie, regiments with Velcade or Kyprolis) and underwent ASCT.
The researchers conclude that sustained MRD negativity after ASCT and a completion of 3 years of Revlimid maintenance may guide the safe discontinuation of maintenance, but more study in clinical trials is needed.
In an analysis of data collected from the MMRF CoMMpass study, Dr. Shan and researchers at the Albert Einstein College of Medicine showed that those who relapse within 12 months of initial therapy have an overall survival (OS) of less than 24 months, regardless of the presence of high-risk features at diagnosis. Out of the 228 myeloma patients classified as functional high-risk myeloma, 63 did not have high-risk chromosomal features specifically–t(4;14), t(14;16), t(14;20), 1q amplification (amp1q), 17p deletion(del17p), or a p53 mutations. The researchers conclude that future studies are needed to confirm if functional high-risk status could be a useful tool for identifying patients who lack chromosomal changes with poor prognosis.
24-hour urine assessments are a key component of International Myeloma Working Group (IMWG) response criteria for myeloma and are requested in clinical trials even if not routinely used in real-world practice. Recent research has suggested a limited role for 24-hour urine assessment if serum immunofixation or free light chain testing is abnormal. In this abstract, Dr. Banerjee and colleagues at the University of Washington analyzed data from 636 patients in the phase 3 STaMINA trial. Outside of situations such as AL amyloidosis where 24-hour urine assessments remain critical or for patients with urine as their only measurable MM biomarker, our results support the removal of 24-hour urine assessments from future iterations of IMWG response criteria.
While there is no cure, the prognosis for myeloma patients has significantly improved, thanks to advances in treatments over the past 10-20 years. The life expectancy for multiple myeloma patients depends on several factors, including the stage of the disease, genetic factors, age, and overall health. One study followed patients for at least 15 years to look at patient- and disease-related factors that may identify those who have a better chance of living 15 years after their diagnosis.
Findings from one abstract of 323 patients in this study, 40 survived at least 15 years. What factors helped predict long-term survival?
Finally, the first day of ASH revealed some promising results on the use of iberdomide in the treatment of newly diagnosed patients who are not eligible for a transplant and those with intermediate and high-risk smoldering multiple myeloma (SMM), an early stage of myeloma that hasn’t yet caused symptoms but is identified by the presence of a serum monoclonal (M) protein of ≥3 g/dL and/or 10% to 60% clonal bone marrow plasma cells.
Iberdomide is a novel oral cereblon E3 ligase modulator (CELMoD™), which is like but more potent than Revlimid, with a dual function: activate the immune system and directly kill myeloma cells.
The first study, which included 75 patients enrolled in an early phase clinical trial, showed the addition of iberdomide combined with Darzalex (daratumumab) and dexamethasone was very effective, with 30 patients achieving MRD negativity.
The second abstract reported findings from an early phase trial that looked at the use of iberdomide alone or in combination with dexamethasone for the treatment of intermediate- or high-risk SMM). Of the 20 patients examined, Dr. Joseph and colleagues from Emory University found that 79% of those with intermediate- or high-risk SMM responded to iberdomide alone or in combination with dexamethasone. Common side effects included headache, abdominal cramping, dyspepsia, and insomnia.
Further studies will continue to evaluate iberdomide for the treatment of early-stages of disease.
Stay tuned tomorrow for additional updates from trials evaluating treatment of patients with newly diagnosed and relapsed/refractory disease.
The final day of ASH gave us an important late-breaking abstract on Darzalex Faspro (daratumumab and hyaluronidase-fihj) in combination with the standard care regimen of Velcade (bortezomib), Revlimid (lenalidomide), and dexamethasone (VRd) in newly diagnosed patients with multiple myeloma (NDMM) who were eligible for an autologous stem cell transplant (ASCT).
Dr. Pieter Sonneveld and colleagues from the Netherlands presented findings from the phase 3 PERSEUS trial that showed the that subcutaneous Darzalex Faspro-based induction, consolidation and maintenance regimen reduced risk of progression or death by 58 percent compared to RVD (Abstract LBA-1).
Results of the trial, which included 709 patients with NDMM who were eligible for ASCT, were simultaneously published in the New England Journal of Medicine (Sonneveld P, NEJM 2023). The researchers reported that the 4-year PFS was 84% with D-VRd compared with 68% with VRd. Significantly more patients achieved higher minimum residual disease (MRD)-negative rates with the addition of Darzalex Faspro (75%) compared to VRd (48%). The overall safety profile of D-VRd was similar to previously reported (Voorhees PM, Lancet Haematol 2023) side effects with D-VRd. The most common (>10 percent) serious grade side effects with D-VRd vs VRd were low white blood cell counts (62% vs 51%), low platelet counts (29% vs 17%), diarrhea (11% vs 8%), and pneumonia (11% vs 6%).
The authors conclude that these results support Darzalex-based quadruplet induction and consolidation regimen and doublet maintenance regimen as a potential new standard of care for transplant-eligible patients with NDMM.
Day 3 was a big day at ASH, with over a dozen abstracts that featured updates on CAR T-cell therapy, bispecific antibodies, the impact of age on transplant outcomes, and novel therapies in early phase clinical trials. Let us break down the key findings for you…
Induction therapy with proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs) followed by high-dose chemotherapy and autologous hematopoietic cell transplantation (ASCT) is considered a standard of care for front-line treatment of patients with newly diagnosed multiple myeloma (NDMM). Some individuals ages 65 and older may have a higher risk of complications and prolonged hospitalizations following ASCT. Therefore, healthcare professionals must carefully assess each patient when recommending ASCT.
Dr. Shohei Mizuno and colleagues from Japan analyzed global registry data that included over 60,000 patients to assess the impact of age on outcomes following ASCT (Abstract 785). Their results showed that patients aged 75 or older had a significantly lower progression free survival (PFS)—that is, the length of time during and after treatment in which a patient is living with a disease that does not get worse and overall survival. The researchers note that incidence of relapse was similar regardless of age and the incidence of death not related to disease progression or treatment failure was low (4%) compared to patients in other age groups (0%, 1%, 2%, and 2% for patients aged 18–39, 40–64, 65–69, and 70–74 ). While ASCT has been generally reserved for patients younger than 65, the authors note that age should not be considered a barrier to receiving this type of treatment.
Dr. Paula Rodríguez Otero and colleagues from Spain presented (Abstract 1028) the final PFS analysis of KarMMa-3 (Rodríguez-Otero et al. NEJM 2023), which showed that a single infusion of the CAR T-cell therapy, Abecma (idecabtagene vicleucel), significantly prolonged PFS and improved response rates in patients with relapsed and refractory multiple myeloma (RRMM) who had received two to four prior lines of therapy, including an immunomodulatory drug (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody, which makes them “triple-class exposed”. The researchers reported that patients treated with Abecma had a median PFS of 13.8 months compared with 4.4 months for standard regimens. Overall response rates (ORR) were higher with Abecma (71%) compared to standard regimens (42%). The authors noted that Abecma side effect profile was consistent with previous reports.
Dr. Jens Hillengass and colleagues from Roswell Park reported (Abstract 1021) follow-up results for patients in two of those groups: Cohort A, who had received between one and three prior lines of therapy (LOT) and whose disease progressed while receiving Revlimid and Cohort B, who had relapsed a year or less after either ASCT or from the beginning of initial treatment if they did not undergo ASCT. The results showed:
Talvey is the first approved bispecific antibody targeting G protein–coupled receptor family C group 5 member D (GPRC5D) for patients with RRMM who have received at least four prior lines of therapy, including a IMiD, a PI, and an anti-CD38 monoclonal antibody. The FDA granted an accelerated approval to Talvey based on initial findings from the MonumenTAL-1 study (Chari A NEJM 2022), which showed that patients who received the 0.8 mg/kg biweekly dose had an overall response rate (ORR) of 74%, similar to those who had received the 0.4 mg/kg biweekly dose. The most common side effects related to treatment with Talvey include cytokine release syndrome (CRS; which is a flu–like syndrome in which a patient experiences fevers, chills, and low blood pressure), immune effector cell-associated neurotoxicity syndrome (neurologic symptoms like confusion, but in some cases patients experience severe symptoms like delirium or seizures), infections, skin and nail disorders such as brittle or discolored nails, and oral toxicities such as dry mouth, difficulty swallowing, and altered taste.
In this presentation, Dr. Ajai Chari and colleagues at Mount Sinai School of Medicine (Abstract 1010) reported that dose modifications of Talvey improved side effects while maintaining responses for patients with RRMM. For this analysis, 9 patients received a starting dose of Talvey at 0.8 mg/kg every 2 weeks, which was reduced to 0.4 mg/kg every 2 weeks following a partial response (PR) or better. A separate group of 10 participants started at 0.8 mg/kg every 2 weeks, which was reduced to 0.8 mg/kg every 4 weeks following a PR or better. The results showed:
The researchers concluded that these data support flexibility to adjust the dosing of Talvey in patients who respond to potentially improve patient experience while maintaining efficacy.
In this presentation, Dr. Jeffrey Matous and colleagues at the Colorado Blood Institute presented (Abstract 1014) initial efficacy and safety results of an early phase trial examined the combination of Talvey with Pomalyst. The results showed rapid, deep responses with the combination of Talvey and Pomalyst in patients with RRMM that had received 2 or more prior lines of therapy. Side effects of the combination were consistent with previous reports of the individual agents:
Dr. Chari and colleagues conclude that these findings support future investigation into the use of combination Talvey and Pomalyst in patients with RRMM.
CELMoDs are a new class of myeloma drugs that work like immunomodulators such as Revlimid and Pomalyst and are orally administered. They also stimulate the immune system and kill myeloma cells directly, even for patients whose myeloma has become resistant to certain treatment. Dr. Paul Richardson and colleagues presented their findings from an early phase trial evaluating mezigdomide (Abstract 1013), an oral CELMoD, in combination with Darzalex and dexamethasone (MeziDd) or Empliciti (elotuzumab) and dexamethasone (MeziEd) in RRMM. Data collected from 45 RRMM patients who received 2-4 prior lines of therapy showed:
Sonrotoclax is a next-generation BCL-2 inhibitor that has shown more potency than venetoclax in preclinical studies in patients who have a translocation of chromosomes 11 and 14 (t(11;14)). In this presentation (Abstract 1011), Dr. Hang Quach and colleagues in Australia reported preliminary findings from an early phase study of sonrotoclax in combination with dexamethasone in 10 RRMM patients with t(11;14). 70% of patients who received the highest dose of sonrotoclax (640mg) achieved a treatment response. 26% of patients experienced severe grade side effects such as increased liver enzymes, diarrhea, low potassium levels, cataracts, and retinal detachment.
Dr. Susan Bal and colleagues at the University of Alabama at Birmingham reported data from an early phase clinical trial with 70 patients that evaluated 5 different doses of BMS-986393, a novel GPRC5D-targeted CAR T-cell therapy for RRMM (Abstract 219). The results showed:
In the next presentation, Dr. Matthew Frigault and colleagues at Harvard Medical School reported data from an early phase trial of CART-ddBCMA, now known as anitocabtagene autoleucel (anito-cel) in 38 patients with RRMM who have received 3 or more lines of therapy (Abstract 1023). CART-ddBCMA has a unique protein, called a D-Domain, that is designed to bind more firmly to BCMA and reduce the risk of side effects such as CRS.
The results showed:
Dr. Aina Calde and colleagues in Spain shared an update on an early phase trial of ARI0002h (Cesnicabtagene Autoleucel), a novel CAR T-cell therapy for RRMM patients (Abstract 1026). Earlier this year, Dr. Calde reported (Oliver-Caldés, Lancet Onc. 2023) that ARI0002h produced durable responses in 30 patients with RRMM who had received at least 2 prior lines of therapy, including a PI, IMiD, and an anti-CD38 antibody, and were refractory to the last line of treatment. This update included 30 additional patients and a longer follow-up of the initial 30 patients treated with ARI0002h.
The results showed:
The final abstract features data from an early phase trial of a novel tri-specific antibody, HPN217, which binds to three targets: BCMA, CD3, and albumin. Researchers note that HPN217 was designed to bind to albumin to extend the length of time the tri-specific antibody can attack myeloma cells and reduce side effects. Dr. Sumit Madan and colleagues from the Banner MD Anderson Cancer Center shared their results of 97 patients with RRMM who had received at least three prior therapies (Abstract 1012). Their findings showed:
Ongoing studies will continue to explore the potential of these novel therapies for patients with RRMM.
Be sure to hear what myeloma experts Dr. Urvi Shah and Dr. Benjamin Diamond, had to say about the day’s presentations here.
Stay tuned for more updates from the final day of ASH 2023!
Day 2 of ASH represented the calm before the storm, as only a couple of abstracts on myeloma were featured on Sunday compared to the more than a dozen presentations that will be highlighted on Day 3. Updates from Day 2 included a real-world comparison of quadruplet versus triplet regimens in standard- and high-risk newly diagnosed multiple myeloma and an assessment of the real-world utilization of autologous stem cell transplantation (ASCT) in newly diagnosed patients.
Preferred treatments for induction therapy (the first in a series of treatments used to treat multiple myeloma) typically consist of three-drug (triplets) or four-drug (quadruplets) regimens given over three to six cycles, each of which typically lasts 3 or 4 weeks. The combination of Revlimid (lenalidomide)-Velcade (bortezomib)-dexamethasone (RVd) is highly effective for patients with NDMM. However, the addition of Darzalex (daratumumab) to RVD (D-RVD) has shown improved depth of response and trend towards a benefit of progression free survival (PFS)—that is, the length of time during and after treatment in which a patient is living with a disease that does not get worse.
In this presentation, Dr. Nisha Joseph and colleagues from Emory University (Abstract 647) analyzed the real-world response rates and long-term outcomes for both standard- and high-risk patients. High-risk disease was defined as having chromosomal alterations including del(17p), t(4;14), and t(4;16). The real-world analysis included 1000 NDMM patients treated with RVD and 326 NDMM patients treated with D-RVD induction therapy. The results showed:
Dr Joseph and colleagues concluded that D-RVD is a highly effective induction regimen that can improve upon outcomes in a historical NDMM population treated with RVD in terms of depth of response and PFS benefit. This analysis provides evidence of benefit with the addition of daratumumab to RVD in increasing depth of response and provides an early glimpse of the promising PFS and OS benefit not only in standard risk patients, but also in patients with high-risk cytogenetic and disease features.
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) remains standard of care in multiple myeloma (MM) in eligible patients and is proven to improve progression free survival. However, some patients do not receive ASCT as part of their treatment. It is well documented that racial and socioeconomic disparities in the use of ASCT exist in myeloma treatment. Barriers to patients receiving ASCT as part of their treatment may include low income, insurance status, and poor access to care at an academic health center. In this presentation (Abstract 532), Dr. Chakra Chaulagain and colleagues at the Cleveland Clinic Florida evaluated data from the National Cancer Database and found that the ASCT opt out rate in real-world is low (approximately 2%) but still represents a missed opportunity to provide standard of care for myeloma patients. Older patients (aged over 60 years), females, African American patients with non-private health insurance (that is, those covered by Medicaid, Medicare, or another government insurance), higher comorbidities, and those with an income of less than $63,000 per year were found to be less likely to receive an ASCT.
Among 43,653 patients evaluated in the study, those treated at non-academic medical centers were less likely to receive ASCT than patients treated at academic facilities. Patients living in the South Atlantic region of the United States were less likely to receive an ASCT compared to other regions of the country. The researchers conclude that their findings reveal significant racial, economic, and geographic variation regarding the use of ASCT across the US which should be further studied. Understanding the barriers to the use of ASCT is crucial for optimizing patient care and tailoring effective interventions.
Be sure to hear what myeloma experts Dr. Nisha Joseph and Dr. Alexander Lesokhin, had to say about the day’s presentations here.
Stay tuned for more updates from day 3 at ASH 2023!
Welcome to our 2023 recap of the latest findings on myeloma treatments reported at the American Society of Hematology (ASH) meeting that kicked off Saturday in San Diego. Highlights from today included real-world data on the use of the bispecific antibody Tecvayli, health-related quality of life findings with the CAR T-cell therapy Abecma, the use of minimum residual disease negativity, iberdomide maintenance therapy, and updates on the treatment of high-risk newly diagnosed disease and smoldering myeloma.
Tecvayli (Teclistamab) was the first “off-the shelf” BCMA-targeted bispecific antibody approved for patients with heavily pretreated relapsed/refractory multiple myeloma (RRMM; that is patients who have received 4 or more lines of therapy), including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 monoclonal antibody. In this Abstract 91, Dr. Danai Dima and colleagues from the Cleveland Clinic reported that treatment with Tecvayli in a real-world setting showed similar responses to the previously reported data from the MajesTEC-1 clinical trial without any new side effects.
Of the 102 patients with RRMM included in Dr. Dima’s analysis, 25% were non-Hispanic Black, 44% had extramedullary disease (EMD; that is, myeloma cells that are growing outside of the bone marrow) and more than 80% (83/102) would not have met the MajesTEC-1 eligibility criteria. Their findings showed:
The authors note that results from the real-world study of the safety and efficacy of Tecvayli were like previously reported data from the MajesTEC-1 clinical trials; however, complete response (CR) rates were lower. The lower CR rates may be due to the fact that a majority of patients evaluated did not meet the eligibility criteria MajestTEC-1 trial population; due to stringent eligibility criteria, patients in clinical trials tend to be less sick and/or have fewer comorbidities than real-world patient population, so outcomes are typically better in clinical trials. Low blood cell counts and infections remain a challenge, thus, close monitoring, supportive care and other measures are important to help patients continue with therapy.
Dr. Michel Delforge and colleagues in Belgium reported findings from a preliminary analysis (Abstract 96) that showed the chimeric antigen receptor T cell (CAR T-cell) therapy Abecma (idecabtagene vicleucel) significantly improved health-related quality of life when compared to standard regimens [that is, Darzalex (dara, daratumumab), Pomalyst (pomalidomide), and dexamethasone; Darzalex, Velcade (bortezomib), and dexamethasone; Ninlaro (ixazomib), Revlimid, and dexamethasone; Kyprolis (carfilzomib) and dexamethasone; or Empliciti (elotuzuamb), Pomalyst, and dexamethasone].
Researchers evaluated data from patient-reported outcomes that were collected as part of the KarMMa-3 trial. In the phase 3 trial, Abecma significantly improved progression-free survival (PFS) and treatment response rates as compared with standard regimens, in patients with triple-class exposed (TCE) RRMM who had received 2–4 prior regimens (Rodriguez-Otero P, et al. N Engl J Med 2023).
Health-related quality of life captures information on the physical and mental health status of individuals, and on the impact of disease and treatment on a patient’s quality of life. The results from the patient-reported outcomes showed statistically significant and clinically meaningful improvements in health-related quality of life including cognitive functioning, fatigue, and pain reduction for patients with RRMM who received Abecma compared with standard treatment regimens. The authors noted that health-related quality of life improvements occurred earlier with Abecma therapy than with standard regimens, starting at approximately 2-3 months following infusion and were sustained for more than 2 years.
A couple of abstracts evaluated the role of minimal residual disease (MRD) as a tool to measure the success of therapy. MRD is an important topic in the field of multiple myeloma, in large part because we have very active treatment regimens that can bring deep and sustained responses to patients, something that was not possible just several years ago. Measuring MRD refers to counting the number of multiple myeloma cells that remain in a patient after a course of therapy is completed. For some patients, achieving MRD negativity (that is, no disease was detected after treatment) is associated with a significantly longer time before disease progression (progression-free survival [PFS]) and overall survival; however, to date, there has been limited information about its clinical meaning in patients treated with CAR T-cell therapy.
In the first presentation, (Abstract 94), Dr. Aintzane Zabaleta and colleagues from Spain found that achieving sustained MRD negativity resulted in significantly prolonged survival of RRMM patients treated with newer immunotherapies such as CAR T-cell therapy and bispecific antibodies (also known as T-cell engagers). The researchers reported that MRD negativity was associated with 88% reduction in the risk of progression and/or death. MRD negative rates were significantly higher in patients treated with CAR T-cell therapy (78%) than bispecific antibody therapy (35%).
In the next abstract, Nizar Bahlis, MD and colleagues from Canada reported (Abstract 338) that Venetoclax (Ven) combined with daratumumab (D) and dexamethasone (d) [VenDd] showed higher rates of MRD-negativity and sustained MRD-negativity compared to bortezomib plus Dd (DVd) in patients with t(11;14)-positive RRMM.
Venetoclax is a potent and selective oral BCL-2 inhibitor with demonstrated anti-myeloma activity in patients with t(11;14)-positive RRMM. The combination of VenDd has shown a high overall response rate and tolerable safety profile in the early phase study. The results in 81 patients showed:
The authors concluded that VenDd treatment showed higher rates of MRD-negativity and sustained MRD-negativity compared to DVd in patients with t(11;14)-positive RRMM.
Maintenance therapy with Revlimid (lenalidomide) is the standard of care following induction therapy and ASCT; however, all patients are at risk of relapse following transplantation, and up to 30% stop Revlimid maintenance therapy due to intolerable side effects. Thus, new treatment options with improved activity and tolerability are needed for maintenance therapy.
Iberdomide is a novel, potent oral cereblon E3 ligase modulator (CELMoD™), which is similar to but more potent than Revlimid, with a dual function: activate the immune system and directly kill myeloma cells by inducing the destruction of proteins that drive cancer growth. Researchers from the Netherlands reported (Abstract 208) that iberdomide maintenance therapy following ASCT showed an improvement in response over time in patients who received IMiD/PI-based induction with or without anti-CD38 antibody therapy and ASCT. Researchers noted that:
The most common serious side effects observed with iberdomide were low blood cell counts, infections, and fatigue.
The researchers conclude that iberdomide represents a novel effective post-ASCT maintenance strategy with a favorable safety profile and superior response improvement at 6 months than what has been observed with Revlimid maintenance. Iberdomide is currently being studied (versus Revlimid) as maintenance therapy following ASCT in a phase 3 trial.
While observation is considered standard of care for smoldering multiple myeloma (SMM) patients who have been identified as having a high risk of progressing to active myeloma, therapeutic intervention at the SMM stage may help delay the progression to MM.
Dr. Ola Landgren, and colleagues from the University of Miami presented the final analysis of the phase 2 CENTAURUS study that showed Darzalex monotherapy demonstrated clinical activity in 123 patients with Intermediate-Risk or High-Risk SMM after a median follow-up of approximately 7 years (Abstract 210).
The researchers examined 3 different dosing schedules (that is, long intense; intermediate; short intense) to determine the optimal schedule of treatment administration for the phase 3 AQUILA study. At a median follow up of 85 months, the study found:
The researchers conclude that this final analysis of CENTAURUS continue to demonstrate the clinical activity of DARA monotherapy in patients with intermediate- or high-risk SMM after a median follow-up of about 7 years, which supports the ongoing phase 3 AQUILA study and future SMM trials.
The final day of ASH gave us some insights into treatment for high-risk patients: those who are newly diagnosed with active myeloma and those who have smoldering multiple myeloma (SMM). Additionally, new data were presented on the use of Sarclisa in patients who relapsed early vs. late as well as on the risk of developing second primary malignancies after treatment with Revlimid.
Patients who have high-risk SMM progress much more rapidly to active myeloma than other SMM patients; some SMM patients may never have active disease. The ASCENT study (ABSTRACT 757) is investigating a treatment strategy aimed at reducing the risk of progression in high-risk SMM patients. A four-drug regimen (Darzalex-Kyprolis-Revlimd-dex [Dara-KRd]) is used as induction and consolidation followed by maintenance with Dara-R. Of the 41 patients who completed the scheduled treatment, 38 remain on study with 90% of patients progression-free at three years.
Several trials have looked at different induction and maintenance strategies for multiple myeloma patients considered to have high-risk disease; that is, patients who have genetic abnormalities that result in a faster relapse than patients who don’t have these abnormalities. High-risk MM was defined as the presence of certain cytogenetic abnormalities; these abnormalities differed between studies but in general included 1q amplification, t(4;14), t(14;16), t(14;20), and/or deletion(17p).
The studies that were conducted included:
In these studies, the time until myeloma progressed in patients was lengthened and was observed with the use of KRd as induction (via retrospective analysis) or extended Dara-VR consolidation (via OPTIMUM) and high MRD negativity rates after consolidation with Isa-KRd (via CONCEPT).
The Myeloma XI study data (ABSTRACT 754) was conducted to assess the impact of Revlimid on the development of second primary malignancies (SPMs) in both patients who had received high-dose melphalan (chemotherapy) and those who did not. In both groups, some patients received Revlimid both at diagnosis and for maintenance therapy, while others only received Revlimid at diagnosis or for maintenance.
In patients who had a stem cell transplant:
For those patients who did not have a stem cell transplant:
Investigators conclude that double-exposure is associated with higher incidence of SPM and while deaths were lower in the groups treated with Revlimid, clinicians should assess each individual’s risk of an SPM before starting Revlimid and have a plan for rapid intervention if needed.
The IKEMA trial (Sarclisa-Kyprolis-dex [Isa-Kd] vs Kyprolis-dex [Kd]) showed that patients with RRMM (who had received 1-3 prior lines of therapy) benefit from the use of isa-Kd with respect to depth of response and prolonged PFS, regardless of whether the relapse was early or late (ABSTRACT 753).
Thanks for following along with our posts this year! To learn more about these data from ASH please register for our upcoming expert session here.
Day 2 of ASH myeloma presentations brought us some updates on the use of FDA-approved treatments, including a closer look on the length of Revlimid maintenance therapy, and the clinical potential of a few investigational therapies for heavily pre-treated patients with myeloma.
Darzalex
There were two presentations that reported findings on patients’ quality of life following Darzalex-containing treatment regimens. Dr. Aurore Perrot and colleagues analyzed quality of life data collected from older (median age: 77 years) patients in the phase 3 MAIA trial, which showed the combination Darzalex-Revlimid-dexamethasone (D-Rd) improved progression-free survival (PFS) in older patients with newly diagnosed multiple myeloma (NDMM) who were not planning to have high-dose chemotherapy and a stem cell transplant vs Rd alone (Abstract 472).
Dr. Perrot reported that older patients treated with D-Rd showed sustained improvements in global health (overall health-related quality of life) and physical functioning, with notable reduction in pain through the duration of therapy. Additionally, a higher percentage of older patients continued on D-Rd longer compared to Rd.
In the next presentation, Dr. Silbermann and colleagues reviewed quality of life data obtained from transplant-eligible NDMM patients in the phase 2 GRIFFIN study which compared Darzalex-Revlimid-Velcade-dexamethasone (D-RVd) with RVd (Abstract 473). The authors found that the addition of Darzalex to RVd resulted in greater improvements in health-related quality of life for patients who continued on Revlimid maintenance treatment following induction and consolidation therapy versus RVd alone, again with a notable reduction in pain symptoms. Overall, these findings further support the addition of Darzalex to RVd in transplant-eligible patients with NDMM without compromise of health-related quality of life.
Tocilizumab has proven to be an effective treatment to lessen the impact of cytokine release syndrome, a common side effect experienced by patients who receive either CAR-T or bispecific monoclonal antibody treatment. Preclinical trials suggest that tocilizumab could prevent the development of cytokine release syndrome without limiting the anti-myeloma activity of treatment. In this presentation, Dr. Suzanne Trudel and researchers shared their findings from a phase 1 study that examined whether a single dose of tocilizumab could reduce cytokine release syndrome in myeloma patients who receive the next-generation bispecific antibody, cevostamab (Abstract 567). The results showed that pretreatment with tocilizumab significantly lowered the percentage of patients who experienced cytokine release syndrome (39% with tocilizumab pretreatment versus 91% without pretreatment) without any impact on anti-myeloma activity. The researchers conclude that the data support additional investigation of the use of tocilizumab pretreatment with the goal of substantially reducing the severity of cytokine release syndrome and potentially enabling the outpatient administration of cevostamab and other bispecific antibodies, compared to current protocols, which call for administration in the hospital setting only.
Optimal Duration of Revlimid Maintenance
Revlimid maintenance after stem cell transplant is standard of care for myeloma patients; however, the optimal length of time of maintenance therapy remains uncertain and may differ in subgroups of patients. Dr. Charlotte Pawlyn and colleagues presented their analysis of data from the Myeloma XI trial which included NDMM patients who were intending to receive high-dose chemotherapy and those who were not (Abstract 570). Their analysis reported clear evidence that continuing Revlimid maintenance beyond 3 years is associated with improved progression free survival, or the time before the disease came back, supporting recent findings from the DETERMINATION and STAMINA studies. There does, however, appear to be a time after stem cell transplant at which continuing maintenance may no longer have ongoing benefit over observation. The current analysis suggests that between 4 and 5 years, the benefit diminished in all patients, and this may occur earlier in the subgroup of patients who were minimal residual disease negative after stem cell transplant. Ongoing long term follow up of this and other studies is needed to define the optimal time point of stopping or continuing maintenance.
Modakafusp alfa
Dr. Dan Vogl and colleagues reported the final safety and efficacy findings from a phase 1 study of Modakafusp alfa, a first-in-class antibody–cytokine fusion protein (immunocytokine), allowing specific delivery of IFN to myeloma cells and immune cells involved in destruction of myeloma cells (Abstract 565). This trial was composed of heavily pre-treated myeloma patients, as participants had received at least 3 prior lines of treatment (including a proteasome inhibitor and one immunomodulatory drug), with 7 median lines of therapy. The results obtained from 30 myeloma patients showed:
The authors conclude that modakafusp alfa has a novel mechanism of action, a manageable safety profile, and encouraging efficacy at 1.5 mg/kg with once-a-week dosing. A phase 2 study to better define the best dosing with the optimal benefit/risk profile is currently enrolling.
Mezigdomide
CELMoDs are a new class of myeloma drugs that work like immunomodulators such as Revlimid and Pomalyst. They also stimulate the immune system and kill myeloma cells directly, even for myeloma that has become resistant to certain treatment. Dr. Paul Richardson and colleagues presented their findings from a phase 1/2 trial evaluating mezigdomide (Abstract 568), an oral CELMoD, alone or in combination with dexamethasone in myeloma patients who had previously received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. The median number of previous treatment regimens was 6. The results showed:
The researchers concluded that mezigdomide + dexamethasone had a manageable safety profile and demonstrated promising efficacy in patients with RRMM, including those with prior BCMA-targeted treatment. Mezigdomide is currently being evaluated in combination with standard treatment regimens in MM as part of a large, ongoing phase 1/2 trial and phase 3 trials in combination with proteasome inhibitors are planned.
BMS-986354
BMS-986354 is a next-generation CAR T-cell treatment that is made with a faster manufacturing process (about 5 or 6 days as opposed to several weeks with currently approved products). Dr. Luciano Costa and researchers reported their findings from the phase 1 CC-98633-MM-001 trial in patients who previously had received an autologous stem cell transplant, proteasome inhibitor, immunomodulatory drug, and an anti-CD38 monoclonal antibody – Darzalex or Sarclisa (Abstract 566). The results showed:
The study continues to enroll patients in the dose-expansion phase.
Stay tuned for more highlights from ASH!