News & Events
Updates in Multiple Myeloma from ASH 2024
Several presentations at the 2024 American Society of Hematology (ASH) Annual Meeting relayed new and updated study data in multiple myeloma (MM), along with significant advancements in the treatment of
- Smoldering multiple myeloma (SMM)
- Prognostic indicators
- Newly diagnosed MM (NDMM)
- Maintenance therapy
- Minimal residual disease (MRD) testing
- Relapsed/refractory MM (RRMM)
- AL amyloidosis
Advances in SMM Management
Ongoing research of SMM treatment is focused on early intervention and observation with monitoring, identification of patients at high risk of progression, and novel treatment approaches. Two studies addressed these issues.
Phase 2 EMN15/HOVON147 Study: KRd vs Rd
- Intervention: Lenalidomide and dexamethasone (Rd) ± carfilzomib (K) as induction therapy
- Patient population: 58 patients with high-risk SMM (HR-SMM) identified by MAYO and/or PETHEMA* criteria
- Efficacy: At a median follow-up of 34 months, KRd significantly improved
- MRD negativity, next-generation flow cytometry [NGF]; 10-5 (57% vs 5%; odds ratio [OR]=0.04, 95% confidence interval [CI], 0.00–0.28; P<0.001)
- Progression-free survival (PFS) (hazard ratio [HR]=0.08, 95% CI, 0.02–0.35; P<0.001)
3-year PFS of 94% vs 40%. MRD negativity after 4 cycles was not significantly better with KRd (37% vs 14%; OR=0.27, 95% CI 0.06–1.12; P=0.07).
- Safety: Although KRd was associated with higher rates of grade 3/4 adverse events (AEs) (74% vs 53%), the combination resulted in fewer treatment discontinuations due to progression (6% vs 36%)
- Takeaway: The authors concluded that the addition of K as induction is “feasible and safe in patients with HR-SMM.” It is important to note that treatment of HR-SMM is still an evolving area where high-risk patients are not predictably identified; the benefit of treatment should outweigh its risks
*MAYO criteria: bone marrow plasma cells ≥10%, serum M protein ≥3 g/dL, and serum free light-chain ratio <0.125 or >8; PETHEMA criteria: presence of ≥95% abnormal plasma cells (presence or absence of CD38, CD56, CD19, and/or CD45), and immunoparesis, defined as a reduction (below the lower normal limit) in the levels of 1 or 2 of the uninvolved immunoglobulins.
Phase 3 AQUILA Study of Daratumumab
- Intervention: Daratumumab vs monitoring
- Patient population: HR-SMM (defined as clonal bone marrow plasma cells [BMPCs] ≥10% and ≥1 risk factor [serum M protein ≥30 g/L, IgA SMM, immunoparesis with reduction of 2 uninvolved Ig isotypes, serum involved:uninvolved free light chain ratio ≥8 and <100, and/or clonal BMPCs >50% to <60%])
- Efficacy: At a median follow-up of 65.2 months,
- PFS was significantly improved with daratumumab (HR=0.49; 95% CI, 0.36–0.67; P<0.0001), with a median PFS not reached in the daratumumab group vs 41.5 months in the active monitoring group
- The overall response rate (ORR) was significantly higher with daratumumab (63.4% vs 2.0%; P<0.0001), with a prolonged median time to first-line MM treatment (not reached vs 50.2 months; HR=0.46; 95% CI, 0.33–0.62; P<0.0001)
- Safety: Grade 3/4 AEs were more common with daratumumab (40.4% vs 30.1%), but the treatment was generally well tolerated, with low discontinuation rates (5.7%). The most common AE was hypertension (5.7% vs 4.6%).
- Takeaway: The authors noted that these findings “strongly support the benefit of early intervention with daratumumab monotherapy vs active monitoring.”
Functional Risk and the Role of 24-Hour Urine Tests
Two studies highlighted important aspects of prognostic indicators: one addressed functional high-risk disease status and the other assessed the value of routine 24-hour urine assessments.
Functional High Risk
Functional high-risk status could be a useful tool for identifying patients with poor prognosis, according to one analysis. Functional high-risk MM, characterized by disease progression or death within a year of initial treatment, is a poor prognostic subset regardless of high-risk cytogenetic features at diagnosis. Of the 228 functional high-risk patients identified from the Multiple Myeloma Research Foundation (MMRF) CoMMpass study, 165 were categorized as high risk and 63 as standard-risk (ie, patients with or without high-risk features [t(4;14), t(14;16), t(14;20), 1q amplification (amp1q), 17p deletion (del17p), TP53 mutations, or ISS-stage III disease]). The median overall survival (OS) was 13.2 months for the standard-risk group and 11.6 months for the high-risk group (P=0.29). Primary refractory disease was prevalent in both groups, with OS significantly shorter for patients with high-risk vs standard-risk disease (5.0 vs 7.8 months; P=0.048).
Despite differences in second-line treatment patterns (standard-risk patients received more triplet regimens; high-risk patients relied on doublets), response rates were similar, with median OS for relapsed patients 27.1 months vs 20.2 months (P=0.22 standard- vs high-risk). Overall, functional high-risk patients had an OS <24 months regardless of the presence of high-risk features at diagnosis.
This study confirms that many high-risk disease features do not present with other known high-risk features at diagnosis. More research into reliably identifying patients with high-risk features is needed.
Urine Testing
A secondary analysis of the BMT CTN 0702 (STaMINA) trial evaluating whether 24-hour urine testing adds value to MM response assessments based on International Myeloma Working Group (IMWG) criteria found that removing 24-hour urine testing requirements from response criteria resulted in changes to <1% of patient responses, with no impact on PFS prediction at any response depth. Median PFS for traditional and urine-free criteria was identical across response categories, including 63.0 months for complete response (CR) and 49.6 months for very good partial response (VGPR). Urine-free IMWG criteria remained highly prognostic for PFS (P=0.006; HR=1.05–1.36).
The authors concluded that urine-free criteria “reduce time, toxicity, and discomfort for patients, particularly those with limited dexterity,” though they also noted that these assessments remain critical in specific cases, such as AL amyloidosis or urine-only measurable disease.
Advances in NDMM Induction Therapy
Several studies highlighted the evolution of induction therapy for improving survival and quality of life for patients with NDMM. Current standard-of-care (SOC) regimens, such as quadruplet therapy incorporating daratumumab or isatuximab with bortezomib-lenalidomide-dexamethasone (VRd), continue to demonstrate superior MRD negativity and PFS regardless of autologous stem cell transplant (ASCT) eligibility, making these regimens an appropriate option for a majority of patients. Data on additional investigational therapies, including teclistamab- and belantamab-based regimens, were also presented.
Phase 3 GMMG-HD7 trial: Isatuximab-VRd
- Intervention: VRd ± isatuximab as induction therapy
- Patient population: Transplant-eligible (TE) NDMM patients
- Efficacy: After a median follow-up of 47 months, compared with VRd alone, isa-VRd:
- Significantly improved PFS (HR=0.70, 95% CI, 0.52–0.94; P=0.0184), with 3-year PFS rates of 83% vs 75%, respectively
- Provided consistent PFS benefits across most baseline characteristics through subgroup analyses, though patients with high-risk cytogenetics and poor performance status showed no significant improvement. Multivariable analysis indicated a benefit for isa-VRd (HR=0.64, 95% CI, 0.47–0.86, P=0.004)
- Takeaway: The MRD negativity benefit with isa-VRd is consistent with previous reports
Phase 3 IMROZ trial: Isatuximab-VRd
- Intervention: VRd ± isatuximab
- Patient population: Transplant-ineligible (TI) NDMM patients
- Efficacy: Compared to VRd alone, isa-VRd
- Achieved higher rates of MRD-negative CR (56% vs 41%; P=0.003; next-generation sequencing [NGS], 10-5) and sustained MRD negativity for ≥12 months (47% vs 24%)
- Had shorter median time to MRD negativity (14.7 months vs 32.8 months)
- Demonstrated greater depth of MRD negativity and more frequent positive-to-negative MRD conversions during maintenance over 36 months, correlating with PFS benefits
- Takeaway: These findings support the benefit of adding isatuximab to VRd as initiation therapy, as well as during maintenance in TI NDMM patients
Investigational Quadruplets
Notable findings regarding investigational quadruplets in NDMM were reported from two studies.
The phase 2 MajesTEC-5 study evaluated the use of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in combination with daratumumab-lenalidomide-dexamethasone (DRd) or D-VRd as induction therapy for patients with TE NDMM. Out of 49 patients, all assessable patients (n=35) achieved MRD negativity (NGF, 10⁻⁵) after completing 3 treatment cycles, with sustained MRD negativity in those completing 6 cycles. Despite a high rate of cytokine release syndrome (CRS) events (65.3%, all grade 1/2), no cases of neurotoxicity or treatment discontinuation due to AEs were reported. The regimens demonstrated robust clinical efficacy with a manageable safety profile, suggesting their potential for improving long-term outcomes in this setting.
Promising results were reported from the phase 1 DREAMM-9 trial of belantamab mafodotin (belamaf) combined with VRd in TI NDMM patients. Across 8 dosing cohorts involving 108 patients, ORR was high, ranging from 71% to 100%, with the CR rate reaching 92% in certain cohorts. MRD negativity (NGS, 10-5) was most notable in higher-dose groups, achieving rates up to 75% in patients with CRs. Ocular events were the most common AE, with grade 3+ keratopathy and visual acuity scale AEs affecting up to 92% of patients in higher-dose groups, though longer dosing intervals reduced the frequency and delayed the onset of these events.
Maintenance Therapy
Also reported at ASH 2024 were findings from two studies on the use of maintenance therapy in MM, including duration of maintenance therapy, role of combination therapies vs single-agent maintenance, MRD status for informing therapy decisions, and the role of novel agents.
Phase 3 AURIGA Trial: Daratumumab + Lenalidomide vs Lenalidomide Alone
- Intervention: Lenalidomide ± daratumumab as maintenance therapy
- Patient population: TE NDMM patients who were MRD-positive after ASCT
- Efficacy: The addition of daratumumab significantly increased the MRD-negative conversion rate (10-5) at 12 months across subgroups, including patients <65 years (49.2% vs 19.7%; OR=3.95, 95% CI, 1.76–8.85) and ≥65 years (52.6% vs 17.5%; OR=5.24, 95% CI, 1.86–14.74), as well as in high-risk cytogenetic subgroups (eg, 43.8% vs 13.3%; OR=5.06, 95% CI, 1.43–17.88). PFS also favored D-R
- Safety: Grade 3/4 treatment-emergent AEs were more frequent with D-R than R, especially in Black patients (75.0% vs 66.7%) and patients <65 years (76.3% vs 63.8%)
- Takeaway: Adding daratumumab to lenalidomide maintenance improves outcomes for NDMM MRD+ patients following ASCT
Teclistamab + Lenalidomide vs Teclistamab vs Lenalidomide
- Intervention: A phase 3 safety run-in study of teclistamab combined with lenalidomide vs teclistamab vs lenalidomide maintenance therapy alone
- Patient population: NDMM patients post ASCT
- Efficacy: 100% MRD-negative CR rate at 12 months in Cohort 1, with all MRD-positive patients achieving MRD-negative status during treatment. Teclistamab was stopped after 13 cycles of treatment if ≥CR was achieved
- Safety: Grade 3/4 neutropenia and infections were common. Neutropenia occurred less frequently with reduced teclistamab dosing schedules; infections followed a similar trend: CRS occurred in 43.6% of patients but was mild (6.4% grade 2; no severe cases), and no immune effector cell-associated neurotoxicity syndrome (ICANS) was reported.
- Takeaway: Teclistamab alone or in combination with lenalidmoide can be safely administered as maintenance therapy following ASCT in NDMM
MRD Testing
MRD and Treatment Cessation
A prospective study evaluated whether discontinuing lenalidomide maintenance therapy after 3 years of sustained MRD negativity (NGF) might be feasible and safe in NDMM patients following ASCT. MRD status was assessed in patients who had achieved stringent CR and then at 6, 12, 24, and 36 months after the initiation of lenalidomide maintenance. Patients who had at ≥3 consecutive MRD-negative results and had received at least 36 months of maintenance discontinued lenalidomide maintenance only if they had also achieved imaging MRD negativity (via PET/CT scan). MRD was performed every 6 months thereafter. At 3 years, 26.3% of patients (N=194) achieved sustained MRD negativity in bone marrow and imaging and discontinued maintenance. Over a median follow-up of 32 months after discontinuation, 96% of these patients remained MRD negative at 6 months, with a gradual decline to 86% at 3 years. Median PFS for this cohort was 74 months (95% CI, 38–104 months).
According to the authors, “sustained MRD negativity after ASCT and completion of 3 years of lenalidomide maintenance may guide the safe discontinuation of maintenance” They also noted, however, that further validation in randomized trials is needed.
MRD After Induction
Several studies demonstrated that sustained MRD negativity with maintenance therapy is associated with improved outcomes. Whether TE or TI, patients showing deeper responses to therapy had prolonged PFS and OS, regardless of therapeutic approach.
- Study: Phase 3 CEPHEUS trial
- Intervention: VRd ± daratumumab in NDMM patients ineligible for or deferring transplant
- Efficacy: At a median follow-up of 58.7 months, compared to VRd alone, dara-VRD
- Improved MRD negativity rates (NGS, 10-5), 60.9% vs 39.4% (OR=2.37; 95% CI, 1.58–3.55; P<0.0001)
- Improved sustained MRD negativity at ≥12 months, 48.7% vs 26.3% (OR, 2.63; 95% CI, 1.73–4.00; P<0.0001)
- Was associated with over 80% of patients remaining progression-free at 54 months
- Takeaway: D-VRd is a potential new SOC for TI NDMM patients
Results from another study suggested that, in NDMM patients treated with quadruplet therapy and ASCT, MRD progression (defined as a ≥1-log10 increase in MRD burden) was associated with a median time of 10.1 months to IMWG-defined progression.
- 78% of patients (N=216) achieved MRD negativity at the 10⁻⁵ threshold (NGS), with 56% ceasing therapy upon confirmed negativity
- MRD progression was identified as an indicator of imminent progression, yielding a 1-year survival free of second-line failure rate of 55.6%, compared to 35% for progression not preceded by MRD progression
- Notably, MRD progression outcomes, including a 2-year OS rate of 78%, were comparable to IMWG-defined progression
The authors concluded that MRD progression challenges reliance on paraprotein-based progression criteria, suggesting the need for earlier intervention and therapies with novel mechanisms of action.
- Study: Phase 3 GMMG-HD7 trial
- Intervention: VRd ± isatuximab
- Patient population: TE NDMM patients
- Efficacy: Compared with VRd alone, isa-VRd resulted in
- Significantly higher MRD negativity rates post induction (55% vs 41%), and continued MRD negativity further improved PFS (HR=0.41, 95% CI, 0.25–0.65; P<0.001)
- Significantly longer PFS in MRD-positive patients (HR=0.64, 95% CI, 0.43–0.96; P=0.03)
- Takeaway: Adding isatuximab to standard VRd therapy achieves deeper responses and improves outcomes in NDMM
An analysis of the GEM2017FIT trial demonstrated that peripheral residual disease (PRD) assessed by mass spectrometry and MRD assessed by NGF have significant prognostic value for PFS in older TI NDMM patients.
- Both PRD and MRD negativity were strongly associated with improved PFS (PRD: HR=29, P<0.0001; MRD: HR=0.31, P<0.0001), with concordant results in 79.6% of cases
- Patients with negative PRD or MRD had superior outcomes across different fitness (Geriatric Assessment in Hematology scale ≤20 or >20) and cytogenetic risk categories, with HR PRD+/MRD+ patients achieving worse outcomes (HR=12, P<0.0001)
According to the researchers, these findings support the integration of PRD evaluation into routine clinical practice to complement MRD, improving risk stratification and informing treatment strategies for older MM patients. Taken together, these findings highlight the role of PRD and MRD negativity as a robust prognostic marker and treatment goal in NDMM.
Biomarkers and Sequencing Strategies for Relapsed MM
Several studies suggested that pretreatment biomarkers and treatment sequencing are important considerations for optimizing therapeutic responses and minimizing toxicity in relapsed MM.
Prior ASCT for Chimeric Antigen Receptor (CAR) T
MM patients had significantly shorter PFS with BCMA-directed CAR T-cell therapy if they had previously received an ASCT, according to one report. A study involving 104 patients indicated that although use of HDM/ASCT did not influence the CR rate (P=0.42), patients treated with prior HDM/ASCT experienced a shorter median PFS with CAR T therapy (9.5 vs 21 months; P=0.01). Multivariate analysis confirmed this association (HR=2.17, 95% CI, 1.25–3.74; P=0.006), independent of other factors such as high-risk cytogenetics or prior treatments. Notably, the timing between HDM/ASCT and CAR T did not influence PFS, and prior HDM/ASCT had no effect on OS, CRS, or ICANS. The authors conclude that these findings may help inform treatment sequencing in MM to optimize outcomes.
Toxicity and Durable Response to CAR T-Cell Therapy
Factors influencing toxicity and durable responses were identified in a comprehensive analysis of pretreatment biomarkers in 108 patients receiving idecabtagene vicleucel (ide-cel) for RRMM. High inflammatory markers (eg, ferritin, IL-6, IL-15; P<0.05) at baseline and elevated plasma cell burden (≥50%; P=0.02) were associated with a higher risk of ICANS, whereas higher cell doses correlated with increased CRS severity (median dose 440 vs 411 × 10⁶; P=0.01). Durable responses (defined as PFS at ≥9 months) were linked to favorable bone marrow profiles, including higher CD4:CD8 ratios and increased cytotoxic natural killer and central memory CD8+ T cells. Conversely, nondurable responses were associated with elevated inflammatory markers, prior BCMA exposure, and high levels of myeloid-derived suppressor cells (P=0.0025). Given these findings, pretreatment evaluation may help optimize patient selection and improve outcomes with ide-cel therapy.
Prior BCMA Exposure for Bispecifics
Findings suggest that response to teclistamab may depend on the timing and type of prior BCMA-directed therapy, offering insights into treatment sequencing in RRMM. In a multicenter study evaluating teclistamab in RRMM, prior exposure to BCMA-directed therapy was associated with a lower ORR (51.4% vs 61.5%; P=0.012) and shorter PFS (median 4.6 vs 8.2 months; P=0.017) than was seen in patients not previously treated with BCMA-directed therapies. Although prior BCMA-directed therapy was not independently predictive of PFS (HR=1.25, 95% CI, 0.95–1.64; P=0.1), waiting >8.7 months between BCMA therapies was linked to superior PFS (8.1 vs 2.5 months; P=0.001). Toxicity profiles were comparable between groups, but grade 3 thrombocytopenia occurred more frequently in the BCMA-exposed cohort (10.7% vs 6.6%; P=0.08).
Early RRMM: Innovations and Emerging Standards
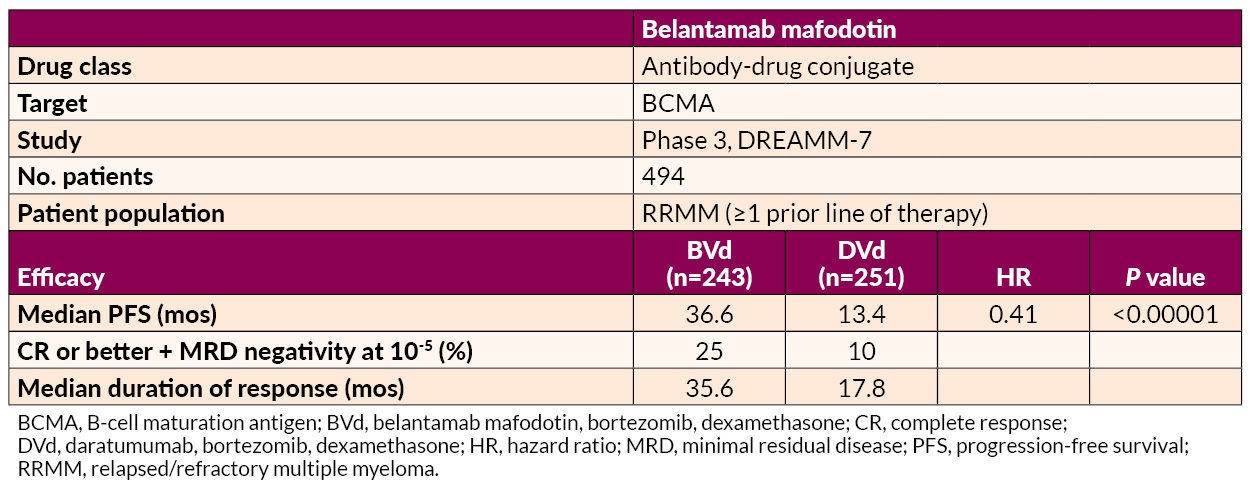
Several studies on the treatment of early RRMM were presented. Belantamab mafodotin–based regimens demonstrated superior PFS to and deeper responses than daratumumab-based combinations, offering a potential new option for first-relapse treatment.
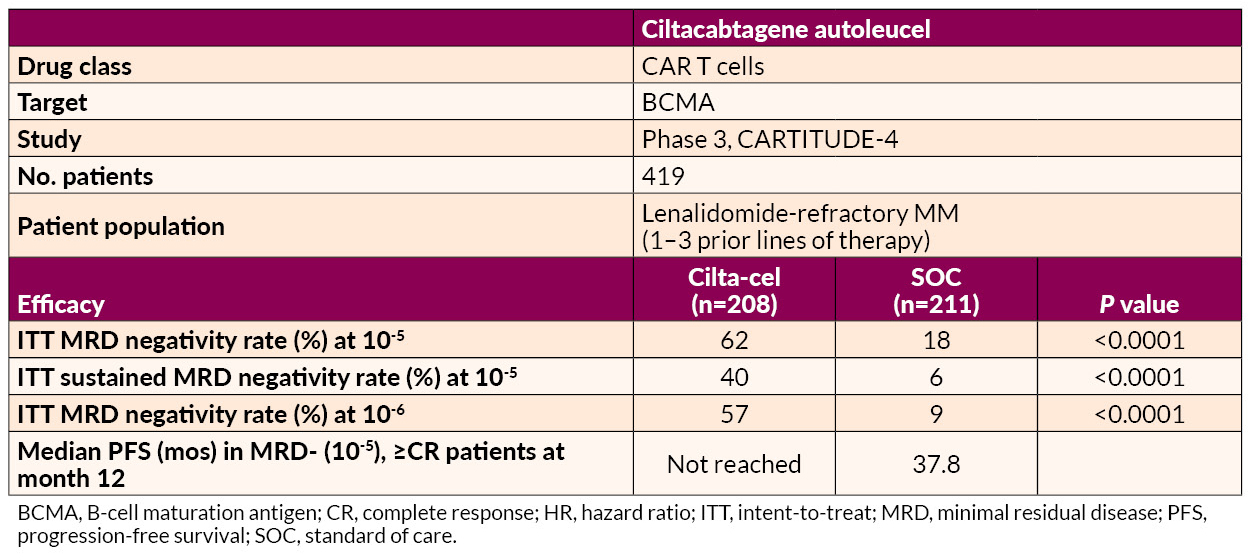
Ciltacabtagene autoleucel (cilta-cel) delivered significant benefits in MRD negativity and prolonged PFS relative to SOC in lenalidomide-refractory patients.
Cereblon E3 Ligase Modulator (CELMoD) and Elranatamab
Mezigdomide in combination with bortezomib or carfilzomib achieved high response rates (up to 85.7%) and durable responses (median PFS up to 17.5 months) across dose-expansion cohorts. Mezigdomide in other novel combinations, such as with agents targeting key oncogenic pathways (eg, MEK and BET inhibitors), displayed promising efficacy with manageable toxicity profiles.
Elranatamab combined with carfilzomib and dexamethasone demonstrated promising efficacy with a 100% ORR and manageable safety signals, including no dose-limiting toxicities in early-phase trials.
Innovative Approaches in Late RRMM Treatment
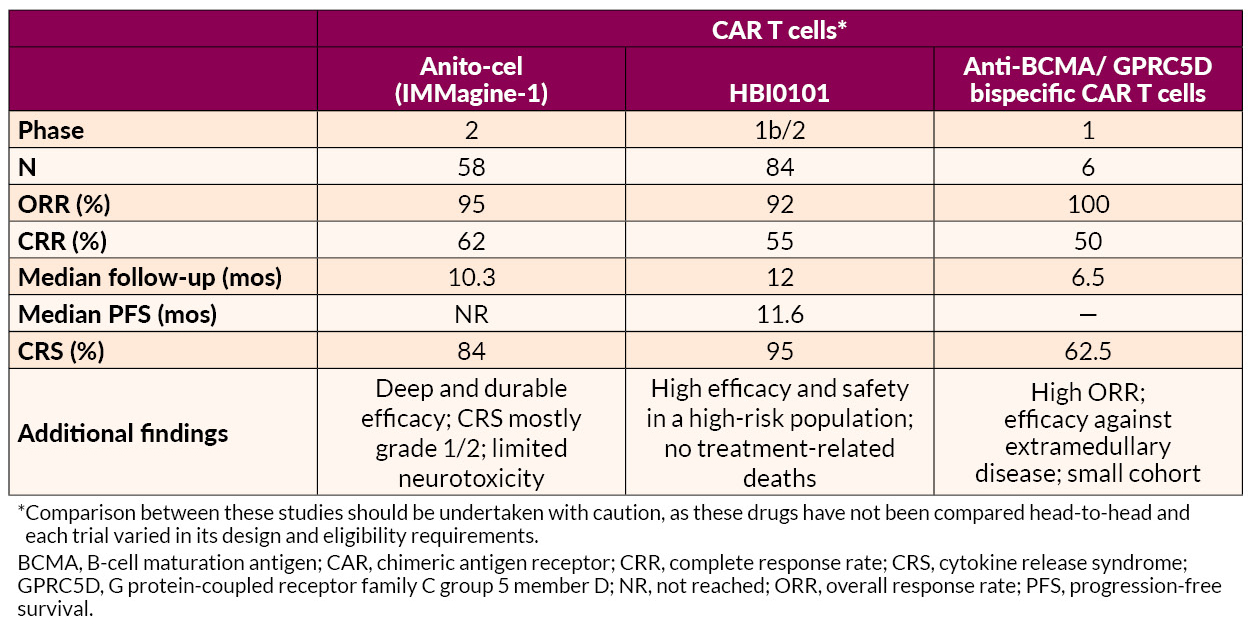
Several novel approaches to late treatment of RRMM with CAR T cells, including BCMA-targeted anitocabtagene autoleucel (anito-cel) and HBI0101 and bispecific antibodies, also showed promise.
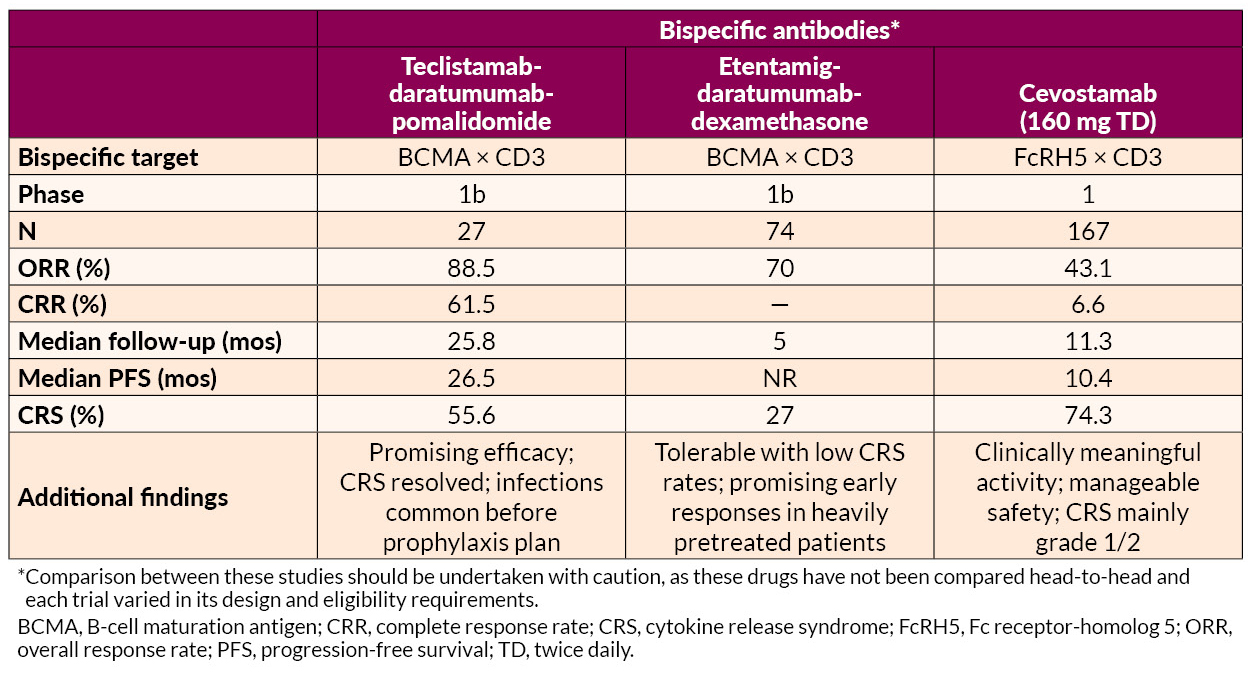
AL Amyloidosis Advances
AL amyloidosis is a plasma cell disorder and occurs in 10% to 15% of MM patients. Studies presented on AL amyloidosis showed benefits for novel drug combinations and CAR T-cell therapy. In addition, one analysis suggested that further development of therapies targeting deposited amyloid fibrils may be useful for addressing cardiac dysfunction associated with AL amyloidosis.
Phase 3 ANDROMEDA trial
- Intervention: Bortezomib + cyclophosphamide + dexamethasone (VCd) ± daratumumab
- Patient population: Newly diagnosed AL amyloidosis patients
- Efficacy: Adding daratumumab to VCd resulted in
- Higher hematologic CR rates (59.5% vs 19.2%; OR=6.03, 95% CI, 3.80–9.58; P<0.0001)
- Prolonged major organ deterioration PFS (MOD-PFS; HR=0.44, 95% CI, 0.31–0.63; P<0.0001)
- Prolonged OS (HR=0.62, 95% CI, 0.42–0.90; P=0.0121)
The median MOD-PFS was not reached for daratumumab-VCd vs 30.2 months for VCd. The 5-year survival rates were 76.1% with daratumumab-VCd vs 64.7% with VCd. Cardiac and renal responses were approximately doubled in the daratumumab-VCd group.
- Safety: Grade 3/4 AEs included lymphopenia and pneumonia
- Takeaway: According to the researchers, the findings reaffirm “this regimen’s position as the only SOC in this difficult-to-treat disease”
Phase 2 ISAMYP Study
- Intervention: Isatuximab + pomalidomide + dexamethasone (IsaPd)
- Patient population: 41 patients with relapsed or suboptimal-response AL amyloidosis
- Efficacy:
- 60% achieving ≥VGPR after 1 cycle
- 80% reaching ≥VGPR after 6 cycles
- 51% achieving CR
Treatment responses were observed early on, with a median time to initial hematologic response of 1 week and ≥VGPR within 4 weeks, including in patients with poor initial responses.
- Safety: Grade 3/4 AEs were observed in 80.5% of patients; most were manageable with dose adjustments or supportive care
- Takeaway: Rapid, deep hematologic responses were achieved with IsaPd
Phase 2 Multicenter Study for t(11;14) AL Amyloidosis
- Intervention: Venetoclax + dexamethasone (ven-D)
- Patient population: Newly diagnosed t(11;14) AL amyloidosis patients
- Efficacy:
- The CR + VGPR rate at 3 months was 58.6%, including CR in 27.6%
- The best hematologic response at any time, a composite of the CR + VGPR, was 62.9%, and the CR rate was 37.1%
- Organ responses at 6 months included improvements in cardiac function (35.0%) and renal function (90.9%)
- Safety: 3 cardiac-related deaths occurred and 6 patients discontinued due to lack of efficacy. Severe AEs such as lymphopenia and liver dysfunction affected 5.6% of patients
- Takeaway: ven-D offers rapid and high-quality hematologic responses with manageable safety risks
Another study found that the novel anti-BCMA-targeted CAR T-cell therapy HBI0101 demonstrated high efficacy and acceptable safety in 16 heavily pretreated patients with relapsed/refractory AL amyloidosis.
- Hematologic responses were present in 94%, with 75% attaining CR
- MRD negativity (flow cytometry; 10-5) was observed in 64% of evaluable patients
- Organ responses occurred in 50% of evaluable cases, with improvements noted in cardiac and renal function
- AEs included
- Grade 3/4 neutropenia (63%)
- Grade 3/4 anemia (31%)
- Manageable CRS in 88% of patients, mostly grades 1–2
The authors noted that earlier intervention before advanced cardiac disease may optimize outcomes, but HBI0101 shows potential to improve organ function and survival in this difficult-to-treat population.
Addressing Cardiac Dysfunction
Further development of therapies targeting deposited amyloid fibrils may be needed to address cardiac dysfunction in AL amyloidosis, according to one report. A single-site retrospective study of 43 patients highlighted the fact that although current systemic AL amyloidosis treatments, including daratumumab- and bortezomib-based regimens, effectively reduce toxic amyloid light chain production, they show limited impact on improving cardiac function. The study showed that 35% of patients experienced clinically significant improvement in cardiac dysfunction, as measured by global longitudinal strain (GLS), but only 10% transitioned from reduced to normal GLS. Most patients (79%) had reduced GLS at baseline, with limited meaningful changes observed across follow-up periods.
—
Jointly provided by the MMRF and RedMedEd.
Support for this activity has been provided through sponsorships from Alexion Pharmaceuticals, Inc.; Pfizer Inc.; and Sanofi US and by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC.

