News & Events
Sarclisa (isatuximab): A New Option for Patients With Newly Diagnosed Myeloma Not Eligible for Transplant
In September 2024, Sarclisa (isatuximab) became the first drug to be approved by the FDA for use with Velcade, Revlimid, and dexamethasone (Isa-VRd) for adult patients who are newly diagnosed with multiple myeloma but unable to receive high-dose chemotherapy and a stem cell transplant commonly used in myeloma treatment.
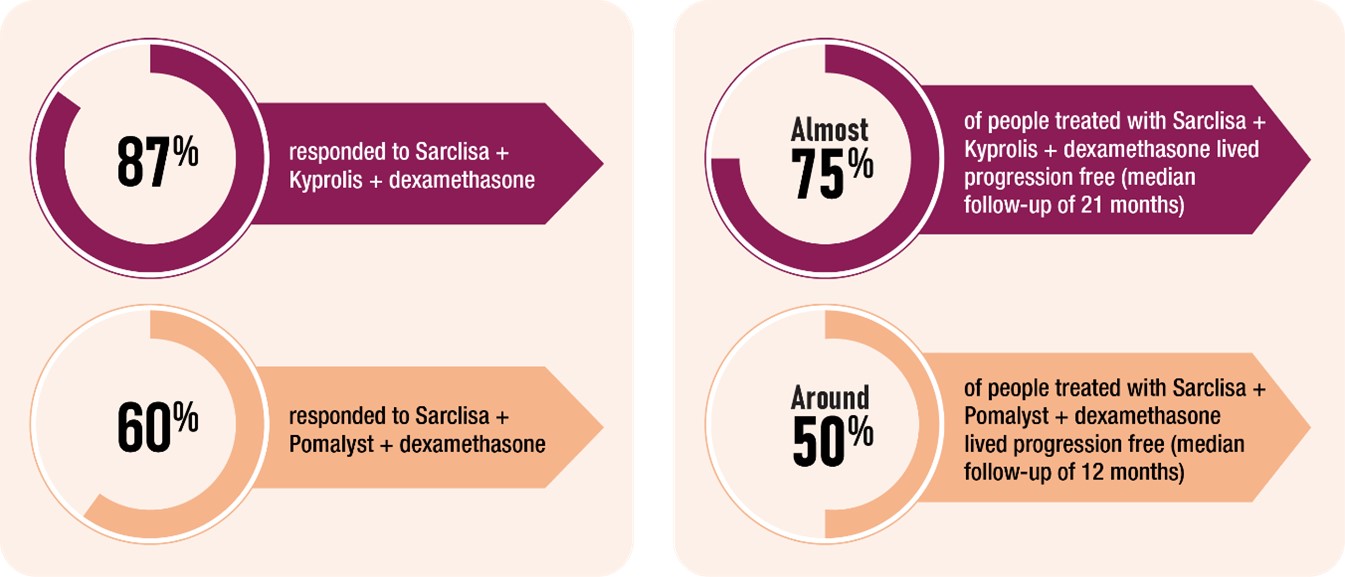
With this new approval, patients with newly diagnosed myeloma have an exciting new treatment option. Previously, Sarclisa had been approved for use as myeloma treatment, in combination with Pomalyst or Kyprolis and dexamethasone, in later lines of therapy.
In clinical trials, most patients who received a combination therapy that included Sarclisa responded to the treatment, and many lived without their myeloma getting worse.
Sarclisa is a monoclonal antibody, a type of immunotherapy agent that is designed to work with your immune system to find, bind to, and destroy myeloma cells in your body.
Importantly, Sarclisa is one of a class of agents that is able to target a specific component of myeloma cells, a protein called CD38. Myeloma treatments that target CD38, which include Darzalex (daratumumab), have several advantages, including the potential for deep responses and longer periods in which myeloma does not progress.
Patients are given Sarclisa by intravenous infusion. Treatment spans cycles of 28 days, in which Sarclisa is administered along with the other agents in the combination therapy. In the first cycle, Sarclisa is usually given weekly. For the remainder of treatment, Sarclisa is usually given every 2 weeks. Currently, studies are looking at whether giving Sarclisa as an injection (instead of an infusion) is an option.
As with most myeloma treatments, there are side effect risks with Sarclisa. Infusion reactions may occur during or shortly after the medication is given. These reactions are most common during the first infusion and can produce fever, chills, nausea, headache, difficulty breathing, low blood pressure, and flushing.
Patients receive medicines before each Sarclisa infusion to help make infusion reactions less frequent and severe. While receiving Sarclisa, patients are monitored for infusion reactions. If a reaction occurs, the infusion can be slowed down or stopped depending on the severity of the reaction.
Low white blood cell, low red blood cell, and low platelet counts occur in most patients. These effects can often be reversed by adjusting the treatment or with supportive measures like transfusions or medications to boost blood cell production. Other side effects include diarrhea, fatigue and nausea, which are typically mild and temporary. Respiratory infections, such as colds or coughs, may develop and usually improve with treatment.
Interested in learning more about Sarclisa? Talk to an MMRF patient navigator at the Patient Navigation Center at 1.888.841.6673 (Monday through Friday, 9:00 am to 7:00 pm ET) or email [email protected].
Additional Resources
MMRF Patient Toolkit: Multiple Myeloma Treatment Overview
All our downloadable resources: Education Resource Hub

