Clinical Trials and Emerging Therapies for Multiple Myeloma
Some people with multiple myeloma may find clinical studies and emerging therapies to be the best option for treatment.
On This Page
Clinical Studies
If you enroll in a clinical study, you will have the opportunity to be among the first to receive the newest drugs and therapies in development. However, it is important to understand that new treatments may be equivalent to, more effective than, or not as effective as existing treatments. They may also have unexpected side effects. That’s why new treatments must be studied in clinical studies first.
Because clinical study participation is a good option for many patients, it is important to understand what they are, how they work, and how to find a study that is right for you. Before a drug is considered for testing in people, it needs to show evidence of activity against myeloma in laboratory and animal studies—these are called preclinical studies.
Different Types of Clinical Studies
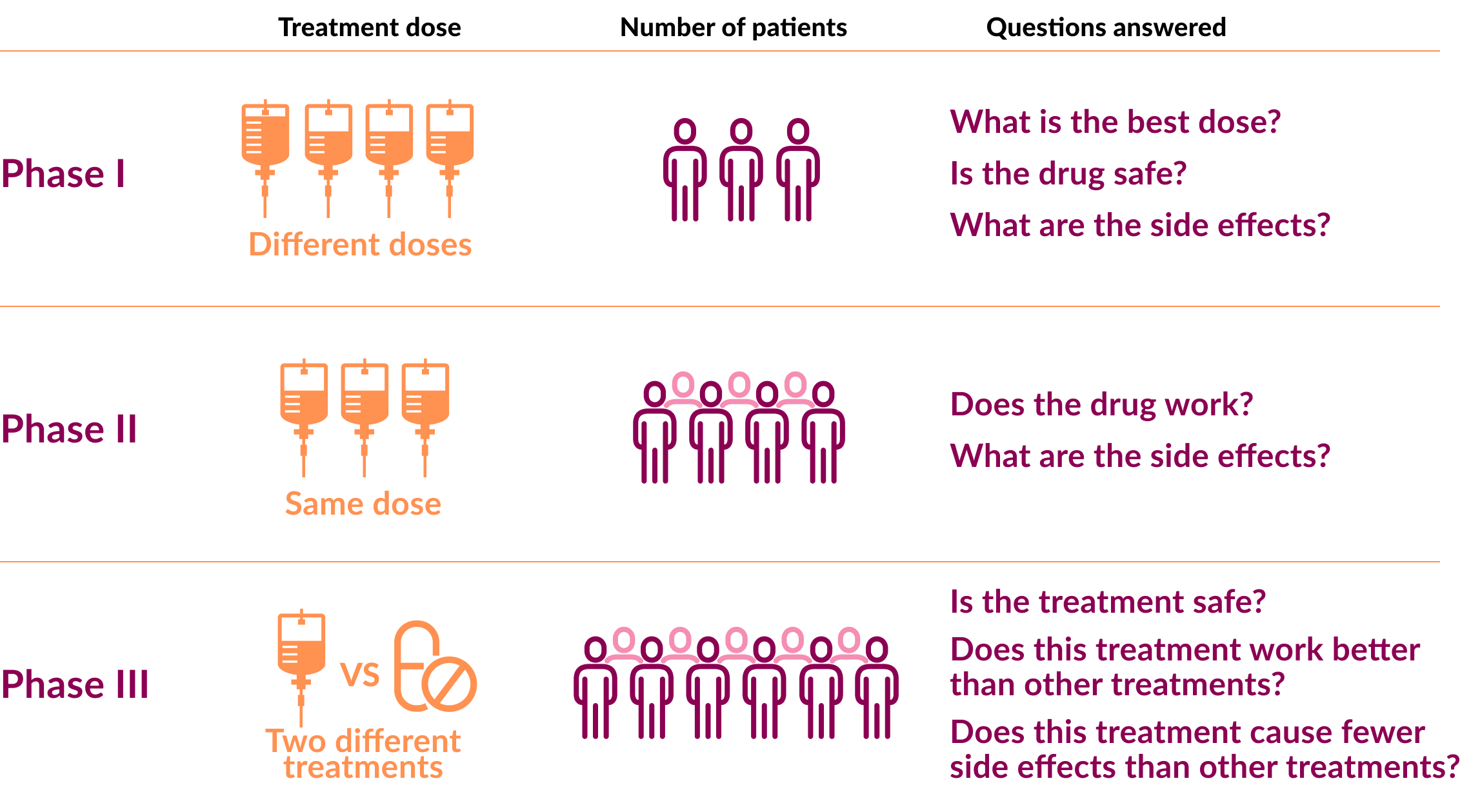
*The FDA approves treatments that are safe, effective, and shown to be better than the standard treatments available. †When no standard treatment is available, the FDA may approve drugs based on study results of phase 2 studies. ‡Conducted to receive FDA approval of new drugs, in most cases.
In addition to the new drugs currently being evaluated in clinical studies, drugs that have been previously approved to treat other diseases are being examined in clinical studies to determine whether they may be effective for treating multiple myeloma.
Some patients worry that participating in a randomized clinical study means that they receive only a placebo and no actual treatment. In fact, all patients in a clinical study receive treatment; one set of patients receives standard treatment (called the standard-of-care group) and the other set of patients receives the new treatment (called the new-treatment group). Physicians and nurses who conduct clinical studies are always the most familiar with the latest research and treatments, and the studies often take place at larger research institutions.
Interested in participating in a clinical study?
Be sure to talk with your doctor about which clinical studies are available and whether one of them may be right for you. If you don’t live near the research institution, it may be possible for your care to be conducted in the office of your own oncologist, or your travel may be limited to a small number of visits to the larger institution.
Emerging Therapies
Emerging multiple myeloma therapies in development*
| Drug Class | Phase 3 | Phase 2 and Phase 1/2 | Phase 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Immunotherapies | Immuno-modulatory Agents | Iberdomide | Cemsidomide | Maplirpacept | QXL138AM | CYT107 | |||
| Mezigdomide | |||||||||
| Naked Antibodies | CM313 | CLN-619 | SAR446523 | ||||||
| Immune checkpoint inhibitor antibodies | Cemiplimab | ||||||||
| Bispecific Antibodies | CM336 | Cevostamab | CM336 | ||||||
| Etentamig | Forimtamig | ||||||||
| Trispecific Antibodies | ISB 2001 | SIM0500 | |||||||
| CAR T-cell Therapies | Anito-cel | CT071 | GC012F | CB-011 | CS1-CAR T | ||||
| Arlo-cel | Zevor-cel | LMY-920 | OriCAR-017 | P-BCMA-ALLO1 | TriPRIL | ||||
| Eque-cel | UF-KURE-BCMA | FT-836 | BMS-986453 | ||||||
| Eque-cel | CBG002 | ||||||||
| Antibody-Drug Conjugates | AZD0305 | HDP-101 | STI-6129 | FOR46-002 | |||||
| NK cell and T-cell Therapies | GIC-102 | IDP-023 | NK-92 | SENTI-202 | |||||
| NY-ESO-1 TCR/IL-15 NK | Kappa CD28 T cells | CAR.70/IL15-transduced CB-NK cells | STI-1492 | ||||||
| Novel Agents | Precision Medicine | Sonrotoclax (in patients with t[11;14]) | Venetoclax (in patients with t[11;14]) | Mirdametinib (in patients with RAS mutations) | Dabrafenib (in patients with BRAF, KRAS, or NRAS mutations) | Trametinib (in patients with BRAF, KRAS, or NRAS mutations) | KTX-1001 (in patients with t[11;14]) | ||
| ABBV-453 (in patients with t[11;14]) | |||||||||
| Other Mechanism of Action | Belumosudil | IDP-121 | Inobrodib | Ruxolitinib | Vactosertib | ||||
| Lisaftoclax | BMS-986158 | OPN-6602 | SX-682 | ||||||
| Tazemetostat |
*Drugs listed are based on a search of www.clinicaltrials.gov with the following search parameters: multiple myeloma, United States, recruiting, not yet recruiting, or active not recruiting (last updated December 2025).
Additionally, research into myeloma therapy is increasingly focusing on personalized medicine—the treatment of patients according to their unique, individual characteristics, such as immune profile and myeloma genetic (DNA) makeup.
Precision medicine uses molecularly targeted therapies—that is, drugs that target genetic errors (DNA mutations) in myeloma cells—and better enables the patient and the healthcare team to choose the myeloma drug(s) that will be most effective.
The MMRF initiated the MMRC Horizon Clinical Trials Program to optimize treatments by maximizing response rates and quality of life, while minimizing side effects in patient populations with high unmet need for safe and effective treatments, beginning in patients with relapsed and refractory myeloma. Horizon, through its design and multi-institutional cooperation, is set up to rapidly answer important research questions for patients whose disease is not always responsive – or durably responsive to currently available treatments.