Norwalk, CT, Dec. 21, 2022 – The Myeloma Investment Fund (MIF), the Multiple Myeloma Research Foundation’s (MMRF) venture philanthropy subsidiary, today announced investments in Luminary Therapeutics, a company using advanced receptor design to improve the depth and durability of patient responses to cell therapy; KAHR, a clinical stage company developing immune-recruiting therapies to address immune escape by tumors; and Telo Therapeutics, a biotechnology company developing second generation inhibitors of nuclear transport against a clinically validated target for multiple myeloma.
These investments mark a significant expansion of the MIF portfolio, bringing the total number of active portfolio companies to nine and the total number of MIF investments to eleven. With these additions, the MIF continues to diversify its range of investments, consistent with its strategic approach to venture philanthropy to accelerate the most promising therapeutic advances for patients with multiple myeloma.
“We are extremely proud to partner with Luminary Therapeutics, KAHR, and Telo Therapeutics, three innovative companies furthering the development of new therapies that have the potential to improve the lives of multiple myeloma patients,” said Peter Kosa, Ph.D., Managing Director of the Myeloma Investment Fund. “By investing in three companies dedicated to developing novel therapies across a variety of innovative new targets and technologies, the MIF remains committed to advancing new treatment options to address unmet needs for multiple myeloma patients.”
The MIF’s investment in Luminary Therapeutics supports the development of Luminary’s BAFF-CAR-T, a first-of-its-kind CAR-T cell therapy that targets three distinct antigens present on multiple myeloma tumors. The MIF’s investment in KAHR supports continued development of DSP107, a dual-targeting fusion protein that activates innate and adaptive immunity by blocking CD47 on cancer cells and utilizing 4-1BB conditional co-stimulatory activation of T-cells. The MIF’s investment in Telo helps advance the company’s next generation nuclear transport inhibitors toward the clinic.
“The three investments in Luminary Therapeutics, KAHR, and Telo Therapeutics are consistent with our mission of accelerating potential life-extending therapies for multiple myeloma patients,” said Michael Andreini, President and CEO of the Multiple Myeloma Research Foundation. “By funding and partnering with innovative companies, we are continuing to advocate for novel technology platforms to be evaluated in the myeloma space where there is still great unmet need.”
For more information, visit myelomainvestmentfund.org, luminarytx.com, kahrbio.com, and telotherapeutics.com.
About the Multiple Myeloma Research Foundation (MMRF)
A pioneer in personalized medicine, the Multiple Myeloma Research Foundation (MMRF) seeks to find a cure for all multiple myeloma patients by relentlessly pursuing innovations that accelerate the development of personalized treatments for cancer. Founded in 1998 by Kathy Giusti, a multiple myeloma patient, and her twin sister Karen Andrews as a 501(c)(3) nonprofit organization, the MMRF has created the benchmark business model around cancer—from data to analytics to the clinic. The MMRF identifies barriers and then finds the solutions to overcome them, bringing in the best partners and aligning incentives in the industry to drive better outcomes for patients. Since its inception, the organization has collected thousands of samples and tissues, opened nearly 100 trials, helped bring more than 15 FDA-approved therapies to market, and built CoMMpass, the single largest genomic dataset in myeloma. Today, the MMRF is building on its legacy in genomics and is expanding into immunotherapy, as the combination of these two fields will be critical to making personalized medicine possible for all patients. The MMRF has raised more than $500 million and directs nearly 90% of the total funds to research and related programs. To learn more, visit www.themmrf.org.
About the Myeloma Investment Fund
The Myeloma Investment Fund (MIF) is a venture philanthropy fund that invests in promising companies, clinical assets, and technologies in oncology to drive the development of new therapies for multiple myeloma. The MIF collaborates closely with portfolio companies to help them advance multiple myeloma research. This evergreen fund is supported entirely by philanthropy; all profits are reinvested back into research for more effective treatments until there is a cure for every patient. For more information, visit MyelomaInvestmentFund.org.
About KAHR
KAHR develops novel dual-targeting fusion protein therapeutics engineered to activate both the innate and the adaptive immune systems simultaneously and localize that response in the tumor microenvironment. KAHR’s lead product candidate, DSP107, is a CD47x41BB targeting compound. DSP107 is being tested in a Phase I/II clinical trial in advanced solid tumors and a Phase Ib clinical trial in blood cancers. KAHR’s preclinical pipeline includes DSP502, a PVRxPD-L1 targeting fusion protein, and DSP216, an HLA-GxCD47 targeting fusion protein. For more information, please visit https://kahrbio.com.
About Luminary Therapeutics
Luminary is a clinical stage allogeneic cell therapy company focused on combining advanced receptor design with superior cell engineering to overcome antigen escape and T cell dysfunction. Luminary was founded by the team from B-MoGen that achieved a successful 5X exit in only three years. Luminary is seeking Series A financing with venture firms or strategic partners to support its first clinical trial and development of its disruptive Universal Receptor that can modulate antigen specificity. For more information visit www.luminarytx.com.
About Telo Therapeutics
Telo Therapeutics is developing orally bioavailable next generation inhibitors of nuclear transport, a critical nexus among many cancer cell signaling pathways. The company’s unique chemistry and distinct mechanism of action result in a favorable safety profile and lead to rapid tumor regressions across multiple cancer types including multiple myeloma, brain, liver, lung, bladder, and prostate. Telo has generated durable complete responses with just two weeks of dosing in xenografts of both multiple myeloma and glioblastoma. Telo is uniquely positioned to unlock the full potential of targeting nuclear transport in multiple myeloma tumors and beyond. Telo is actively raising a Series A financing to advance its small molecule to Investigational New Drug (IND) and run a Phase 1 proof-of-concept trial. Telo is targeting first patient dosing in 2H of 2024.
###
Multiple Myeloma Research Foundation Media Contact:
C.J. Volpe
Director, PR and Communications
Multiple Myeloma Research Foundation (MMRF)
203.652.0453
[email protected]
The final day of ASH gave us some insights into treatment for high-risk patients: those who are newly diagnosed with active myeloma and those who have smoldering multiple myeloma (SMM). Additionally, new data were presented on the use of Sarclisa in patients who relapsed early vs. late as well as on the risk of developing second primary malignancies after treatment with Revlimid.
Patients who have high-risk SMM progress much more rapidly to active myeloma than other SMM patients; some SMM patients may never have active disease. The ASCENT study (ABSTRACT 757) is investigating a treatment strategy aimed at reducing the risk of progression in high-risk SMM patients. A four-drug regimen (Darzalex-Kyprolis-Revlimd-dex [Dara-KRd]) is used as induction and consolidation followed by maintenance with Dara-R. Of the 41 patients who completed the scheduled treatment, 38 remain on study with 90% of patients progression-free at three years.
Several trials have looked at different induction and maintenance strategies for multiple myeloma patients considered to have high-risk disease; that is, patients who have genetic abnormalities that result in a faster relapse than patients who don’t have these abnormalities. High-risk MM was defined as the presence of certain cytogenetic abnormalities; these abnormalities differed between studies but in general included 1q amplification, t(4;14), t(14;16), t(14;20), and/or deletion(17p).
The studies that were conducted included:
In these studies, the time until myeloma progressed in patients was lengthened and was observed with the use of KRd as induction (via retrospective analysis) or extended Dara-VR consolidation (via OPTIMUM) and high MRD negativity rates after consolidation with Isa-KRd (via CONCEPT).
The Myeloma XI study data (ABSTRACT 754) was conducted to assess the impact of Revlimid on the development of second primary malignancies (SPMs) in both patients who had received high-dose melphalan (chemotherapy) and those who did not. In both groups, some patients received Revlimid both at diagnosis and for maintenance therapy, while others only received Revlimid at diagnosis or for maintenance.
In patients who had a stem cell transplant:
For those patients who did not have a stem cell transplant:
Investigators conclude that double-exposure is associated with higher incidence of SPM and while deaths were lower in the groups treated with Revlimid, clinicians should assess each individual’s risk of an SPM before starting Revlimid and have a plan for rapid intervention if needed.
The IKEMA trial (Sarclisa-Kyprolis-dex [Isa-Kd] vs Kyprolis-dex [Kd]) showed that patients with RRMM (who had received 1-3 prior lines of therapy) benefit from the use of isa-Kd with respect to depth of response and prolonged PFS, regardless of whether the relapse was early or late (ABSTRACT 753).
Thanks for following along with our posts this year! To learn more about these data from ASH please register for our upcoming expert session here.

I was diagnosed with multiple myeloma in December of 2010 and immediately began induction therapy. I had a stem cell transplant in July of 2011. While in the hospital post-transplant, my dad suggested that we find a myeloma walk, which led us to the MMRF. That was the beginning of “Team Tracy” and our connection to the MMRF.

I was 50 years old when I was diagnosed, and having an incurable cancer diagnosis set me apart from everyone I knew. It was important that I be around other people I could identify with, but at the same time feel the love from my team! We attended our first event in September of 2011, but I didn’t walk very far. By 2012, I completed the walk! I feel that Team Tracy and the MMRF 5k are all about connecting. The walk/run allows us to connect with our family and friends, along with other members of the myeloma community. From our first interaction, the MMRF community made me feel welcome and supported. Now I feel like a valued member of the MMRF team! We especially love competing with Andy’s Angels and dearly miss our beloved friend Andrea. Andrea was an amazing person and fighter. We participate every year because the MMRF counts on our support to continue the great work that they do to help all myeloma patients.

Receiving the Spirit of Hope Award is an acknowledgement of how important Team Tracy is to the MMRF and their mission. Our team members are impressed by the MMRF’s achievements and are happy to be part of this fantastic organization. I know they will all be proud to share this award.

Since my diagnosis, I have had several friends receive cancer diagnoses, and helping them through these terrible times has given me strength. Six years ago, I began exercising daily, and this has helped tremendously with my mood and attitude. The gratitude that I feel for my continued good health and the additional years with my family has made me a more thoughtful person.
One day at a time is the best way to manage anxiety. During the darkest times—maybe even get through the next hour. Also, lately I’ve been thinking—don’t sweat the small stuff. And of course—count your blessings!
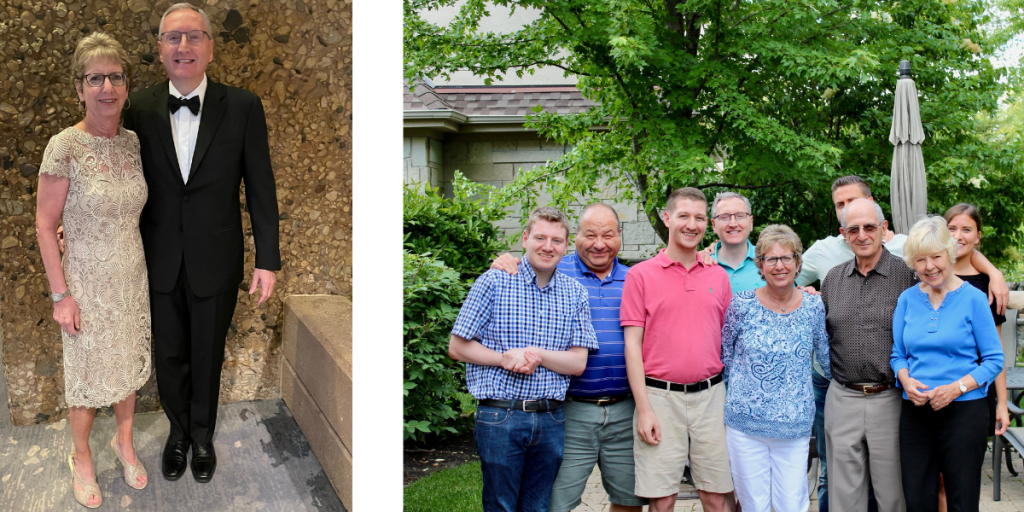
A few of the Team Tracy members have been to every Walk/Run that we have participated in. That is a major achievement! Everyone who signed up for the event in 2022 came to Montrose Harbor even through a torrential downpour! Team Tracy is not just the members of our race day team, but includes everyone that donates. I am touched and always surprised by how many people care about me and want to help the MMRF reach their goal of a cure for MM. The MMRF has become important to all the Team Tracy supporters, and we are honored to receive this award.
The Multiple Myeloma Research Foundation is delighted to recognize Tracy Safron as the MMRF Spirit of Hope Honoree at the 2023 MMRF Team for Cures: Chicago Walk/Run.
This award is presented at every Walk/Run to a patient, caregiver, or family who inspires hope through their resilience, perseverance, and dedication to the MMRF and its mission.
View Tracy’s page to read her story and join the Team here.

When my wife Ardy was diagnosed with multiple myeloma, we were active in the local multiple myeloma support groups. Through these groups, we learned of organizations such as the MMRF that were helping to raise funds and awareness. Ardy was active in the MMRF Walk/Run and participated while she was going through treatments.

We wanted to support MMRF and be supported by the MM community while Ardy was going through treatment. It allowed us to raise funds, build relationships, and help others understand what multiple myeloma is. We also wanted to support others who were going on this journey. After Ardy passed away in 2013, the walk/run became a way to honor her and bring together our community in Ardy’s name.

Spirit of Hope
The Spirit of Hope Award would be the opportunity to honor Ardy, her spirit, the spirit of the multiple myeloma community, the spirit of the hopefulness we can have on this earthy journey, and the hope that we have for the eternal journey.
Faith. Faith and strength for today and great hope for tomorrow. Ardy lost her eyesight as a side effect of the treatment, and that impacted her perspective. We have stories about the caring medical staff (many examples) while being treated; the support of the people in the MM community, friends, and family; the grief process after her loss; and so much more.
Great is thy faithfulness ….
Strength for today and great hope for tomorrow
Fear not for I am with you…
There are so many others who are more worthy of this award. Ardy would not want to draw attention to her struggle, but would want the Lord glorified and magnified because of her challenge. I want the attention on the difference MMRF is having in helping the MM community and the Germann family. You make it easy to come together as a community to provide hope for the future in this life for so many.
The Multiple Myeloma Research Foundation is delighted to recognize the Ardy Germann Team as the MMRF Spirit of Hope Honoree at the 2023 MMRF Team for Cures: Twin Cities Walk/Run.
This award is presented at every Walk/Run to a patient, caregiver, or family who inspires hope through their resilience, perseverance, and dedication to the MMRF and its mission. Donate to Peter Germann’s 2023 Walk/Run fundraising page to accelerate a cure today!
Day 2 of ASH myeloma presentations brought us some updates on the use of FDA-approved treatments, including a closer look on the length of Revlimid maintenance therapy, and the clinical potential of a few investigational therapies for heavily pre-treated patients with myeloma.
Darzalex
There were two presentations that reported findings on patients’ quality of life following Darzalex-containing treatment regimens. Dr. Aurore Perrot and colleagues analyzed quality of life data collected from older (median age: 77 years) patients in the phase 3 MAIA trial, which showed the combination Darzalex-Revlimid-dexamethasone (D-Rd) improved progression-free survival (PFS) in older patients with newly diagnosed multiple myeloma (NDMM) who were not planning to have high-dose chemotherapy and a stem cell transplant vs Rd alone (Abstract 472).
Dr. Perrot reported that older patients treated with D-Rd showed sustained improvements in global health (overall health-related quality of life) and physical functioning, with notable reduction in pain through the duration of therapy. Additionally, a higher percentage of older patients continued on D-Rd longer compared to Rd.
In the next presentation, Dr. Silbermann and colleagues reviewed quality of life data obtained from transplant-eligible NDMM patients in the phase 2 GRIFFIN study which compared Darzalex-Revlimid-Velcade-dexamethasone (D-RVd) with RVd (Abstract 473). The authors found that the addition of Darzalex to RVd resulted in greater improvements in health-related quality of life for patients who continued on Revlimid maintenance treatment following induction and consolidation therapy versus RVd alone, again with a notable reduction in pain symptoms. Overall, these findings further support the addition of Darzalex to RVd in transplant-eligible patients with NDMM without compromise of health-related quality of life.
Tocilizumab has proven to be an effective treatment to lessen the impact of cytokine release syndrome, a common side effect experienced by patients who receive either CAR-T or bispecific monoclonal antibody treatment. Preclinical trials suggest that tocilizumab could prevent the development of cytokine release syndrome without limiting the anti-myeloma activity of treatment. In this presentation, Dr. Suzanne Trudel and researchers shared their findings from a phase 1 study that examined whether a single dose of tocilizumab could reduce cytokine release syndrome in myeloma patients who receive the next-generation bispecific antibody, cevostamab (Abstract 567). The results showed that pretreatment with tocilizumab significantly lowered the percentage of patients who experienced cytokine release syndrome (39% with tocilizumab pretreatment versus 91% without pretreatment) without any impact on anti-myeloma activity. The researchers conclude that the data support additional investigation of the use of tocilizumab pretreatment with the goal of substantially reducing the severity of cytokine release syndrome and potentially enabling the outpatient administration of cevostamab and other bispecific antibodies, compared to current protocols, which call for administration in the hospital setting only.
Optimal Duration of Revlimid Maintenance
Revlimid maintenance after stem cell transplant is standard of care for myeloma patients; however, the optimal length of time of maintenance therapy remains uncertain and may differ in subgroups of patients. Dr. Charlotte Pawlyn and colleagues presented their analysis of data from the Myeloma XI trial which included NDMM patients who were intending to receive high-dose chemotherapy and those who were not (Abstract 570). Their analysis reported clear evidence that continuing Revlimid maintenance beyond 3 years is associated with improved progression free survival, or the time before the disease came back, supporting recent findings from the DETERMINATION and STAMINA studies. There does, however, appear to be a time after stem cell transplant at which continuing maintenance may no longer have ongoing benefit over observation. The current analysis suggests that between 4 and 5 years, the benefit diminished in all patients, and this may occur earlier in the subgroup of patients who were minimal residual disease negative after stem cell transplant. Ongoing long term follow up of this and other studies is needed to define the optimal time point of stopping or continuing maintenance.
Modakafusp alfa
Dr. Dan Vogl and colleagues reported the final safety and efficacy findings from a phase 1 study of Modakafusp alfa, a first-in-class antibody–cytokine fusion protein (immunocytokine), allowing specific delivery of IFN to myeloma cells and immune cells involved in destruction of myeloma cells (Abstract 565). This trial was composed of heavily pre-treated myeloma patients, as participants had received at least 3 prior lines of treatment (including a proteasome inhibitor and one immunomodulatory drug), with 7 median lines of therapy. The results obtained from 30 myeloma patients showed:
The authors conclude that modakafusp alfa has a novel mechanism of action, a manageable safety profile, and encouraging efficacy at 1.5 mg/kg with once-a-week dosing. A phase 2 study to better define the best dosing with the optimal benefit/risk profile is currently enrolling.
Mezigdomide
CELMoDs are a new class of myeloma drugs that work like immunomodulators such as Revlimid and Pomalyst. They also stimulate the immune system and kill myeloma cells directly, even for myeloma that has become resistant to certain treatment. Dr. Paul Richardson and colleagues presented their findings from a phase 1/2 trial evaluating mezigdomide (Abstract 568), an oral CELMoD, alone or in combination with dexamethasone in myeloma patients who had previously received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. The median number of previous treatment regimens was 6. The results showed:
The researchers concluded that mezigdomide + dexamethasone had a manageable safety profile and demonstrated promising efficacy in patients with RRMM, including those with prior BCMA-targeted treatment. Mezigdomide is currently being evaluated in combination with standard treatment regimens in MM as part of a large, ongoing phase 1/2 trial and phase 3 trials in combination with proteasome inhibitors are planned.
BMS-986354
BMS-986354 is a next-generation CAR T-cell treatment that is made with a faster manufacturing process (about 5 or 6 days as opposed to several weeks with currently approved products). Dr. Luciano Costa and researchers reported their findings from the phase 1 CC-98633-MM-001 trial in patients who previously had received an autologous stem cell transplant, proteasome inhibitor, immunomodulatory drug, and an anti-CD38 monoclonal antibody – Darzalex or Sarclisa (Abstract 566). The results showed:
The study continues to enroll patients in the dose-expansion phase.
Stay tuned for more highlights from ASH!
Welcome to our 2022 recap of the latest updates on myeloma treatments reported at the American Society of Hematology (ASH) meeting that kicked off Saturday in New Orleans. Some of the studies presented provided updates to data previously presented at meetings earlier this year and late last year. Let’s dig into a big day of updates in myeloma treatment.
Tecvayli
Tecvayli is the first “off-the shelf” BCMA-targeted bispecific antibody approved for patients with heavily pretreated relapsed refractory multiple myeloma (RRMM). Dr Emma Searle and colleagues presented results from phase 1 MajesTEC-2 trial on 32 relapsed/refractory myeloma patients treated with Tecvayli-Darzalex-Revlimid, the first fully immune-based triplet treatment combination (ABSTRACT 160). The median prior lines of therapy were 2 (range of 1-3), and 31% had received a CD38 monoclonal antibody. Results showed that 93.5% of patients responded to treatment:
The authors conclude that Tecvayli-Darzalex-Revlimid was well tolerated, with a safety profile consistent with Tecvayli or Darzalex-Revlimid individually. Promising response rates rarely seen in this population supports the potential for this combination to have enhanced early disease control through the addition of Tecvayli. The randomized phase 3 MajesTEC-7 study will compare Tecvayli-Darzalex-Revlimid versus the combination of Darzalex-Revlimid-dexamethasone in newly diagnosed multiple myeloma patients not eligible for or not intending to pursue autologous stem cell transplant as initial treatment.
Talquetamab
Talquetamab is a first-in-class, T-cell redirecting bispecific antibody targeting GPRC5D on myeloma cells and CD3 receptors on T cells. Last week Janssen submitted a Biologics License Application to the U.S. Food and Drug Administration for talquetamab for the treatment of patients with RRMM. Dr. Ajai Chari and researchers presented results from a phase 2 study of talquetamab as administered as an injection as opposed to an infusion which is how most other bispecifics are given at 2 different doses (0.405 mg/kg weekly and 0.8 mg/kg every other week) in relapsed/refractory patients (ABSTRACT 157). Eligible patients had RRMM or were intolerant to standard therapies. Patients had received at least 3 or more lines of prior therapy or were double refractory to a proteasome inhibitor such as Velcade and/or Kyprolis and an immunomodulatory drug such as Revlimid and/or Pomalyst. Prior treatment with a CD38 monoclonal antibody was allowed with a 90-day washout; prior BCMA-directed therapies were permitted. The results showed:
The authors conclude that talquetamab shows highly promising efficacy in a heavily pretreated RRMM patient population. An ongoing phase 3 study is comparing talquetamab with approved therapies.
Elranatamab
Updated results were presented on the MagnetisMM-1 trial, a phase 1 trial investigating the safety and efficacy of the BCMA-targeted bispecific antibody elranatamab as a single agent. Fifty-five patients who had been exposed to a median of 6 prior treatments (91% no longer responded to three classes of standard therapies; 24% had received prior BCMA-targeted therapy) received elranatamab subcutaneously (via injection) (ABSTRACT 158). Dr Noopur Raje and investigators reported:
Dr Nizar Bahlis presented results from the MagnetisMM-3 trial (ABSTRACT 159), a phase 2 study of elranatamab in patients with RRMM (exposed to at least one proteasome inhibitor, one immunomodulatory drug, and one anti-CD38 antibody). One hundred and twenty-three patients with relapsed/refractory myeloma (exposed to at least 6 prior lines of therapy) were treated with Darzalex (subcutaneously) and differing doses of talquetamab. Results showed:
The researchers suggest that subcutaneous elranatamab is efficacious and has a manageable safety profile in patients with triple-class- and penta-drug refractory MM and no prior BCMA-targeted treatment. These results support continued development of elranatamab for pts with MM.
Alnuctamab
Alnuctamab is a bispecific antibody that targets BCMA on myeloma cells and CD3 receptors on T cells that is being evaluated in a phase 1 study for patients who have been exposed to at least one proteasome inhibitor, one immunomodulatory drug, and one anti-CD38 antibody (ABSTRACT 162). Results collected from 55 patients treated with alnuctamab found:
Cytokine release syndrome and low red and white blood counts were the most common side effects, which is consistent with other similar therapies.
RG6234
Dr Carmelo Carlo-Stella reported findings from a phase 1 study of RG6234, a bispecific antibody targeting GPRC5D, an orphan receptor expressed on MM cells with limited expression in other tissues, and CD3 receptors on T cells (ABSTRACT 161). One hundred and five patients with relapsed/refractory myeloma (exposed to at least a proteasome inhibitor and an immunomodulatory drug) were treated with differing doses of RG6234 (subcutaneously or intravenously). Results showed:
Dr. Paul Richardson from the Dana-Farber Cancer Institute presented updated data from the ICARIA-MM phase 3 trial which compared Sarclisa-Pomalyst-dexamethasone with Pomalyst- dexamethasone in 307 patients with relapsed or refractory myeloma (Abstract 247). Sarclisa is an antibody in the same class as Darzalex. The initial results from this trial were the basis for the approval of Sarclisa in combination with Pomalyst-dexamethasone.
In the follow-up analysis presented by Dr. Richardson, the findings showed how patients fared on subsequent therapies after no longer being on Pomalyst-dexamethasone with or without Sarclisa:
The researchers conclude that immediate use of an anti-CD38 monoclonal antibody such as Darzalex with currently available combinations appears to be less effective following treatment with Sarclisa-Pomalyst-dexamethasone. These findings will help guide the best sequencing of treatment for patients.
Abecma
Patients with MM who relapse early after frontline therapy with autologous stem cell transplant (ASCT) have a poor prognosis. In the pivotal phase 2 KarMMa study, treatment with the BCMA-directed chimeric antigen receptor (CAR) T cell therapy Abecma resulted in frequent, deep, and durable responses in patients who were triple-class exposed and refractory to last treatment. In this presentation, Dr Krina Patel presented data collected from 37 patients who were treated with Abecma after early relapse within 18 months of frontline therapy with ASCT and Revlimid maintenance (Abstract 361). The results showed:
The researchers conclude that these results support a favorable clinical benefit-risk profile of Abecma and its potential use in earlier lines of treatment.
BMS-986393
CAR T cell therapies targeting B‑cell maturation antigen (BCMA) have resulted in unprecedented responses in RRMM; however, relapses are frequent, and the development of new approaches is needed. Dr Susan Bal and colleagues presented interim results from a phase 1 dose-finding study of BMS-986393, a GPRC5D-targeted CAR T cell therapy, in patients with RRMM (Abstract 364). Patients enrolled in the trial had at least 3 prior lines of therapy containing a proteasome inhibitor, an immunomodulatory agent, an anti-CD38 therapy, and, unless ineligible, a stem cell transplant. The results showed:
These results appear promising, especially for patients who relapse following BCMA-targeted treatment. The dose-finding trial is ongoing and additional updates will be presented at future meetings.
GC012F
GC012F is a new, dual-targeting CAR-T cell therapy that binds to BCMA and CD19 on myeloma cells and is manufactured within 24 hours of removal of plasma from a patient. Dr Juan Du and colleagues reported their findings from a phase 1 trial of GC012F in high-risk, transplant-eligible patients with NDMM (Abstract 366). Patients were considered high-risk if they had one or more of the following features: R-ISS-2 or-3; del17p, t (4;14), t (14;16), or 1q21amp ≥ 4 copies; extramedullary disease; IgD or IgE subtype; LDH > the upper limit of normal; or any of the high-risk definition of mSMART3.0. The results showed:
The authors conclude that these promising preliminary results require further assessment of GC012F for transplant-eligible NDMM with more patients and longer follow-up.
Racial disparities exist in incidence and survival from myeloma and other blood cancers, and these differences are driven by multiple factors the most influential likely being access to clinical trials. Black patients only make up 5-7% of participants in cancer clinical trials, despite accounting for 14% of the population in the United States and more than 20% of multiple myeloma patients. Dr Shakira Grant presented two abstracts (Abstract 360 and Abstract 363) in Patient Representation and Equity in Hematologic Malignancies. Dr Grant and researchers conducted focus groups with Black patients with MM and interviewed hematologists to identify factors that influence Black participant enrollment in hematology-focused clinical trials).
Dr Grant and colleagues reported that the relationship between the patient and their hematologist is one of the most influential drivers of whether Black patients are given an opportunity to participate in clinical trials. Additional factors include 1) race and socioeconomic status-based bias and stereotyping on the part of hematologists, 2) the legacy of medical mistrust among Black persons, 3) geographic barriers, and 4) the use of stigmatizing languages such as the word “trial.”
Researchers reported that addressing disparities in clinical trial enrollment among Black participants requires numerous interventions such as
Increasing equitable access to clinical trials and ensuring our own research efforts are representative of the broader multiple myeloma population in the US are priorities for the MMRF, reflected in our most recent strategic plan.
Dr Shaji Kumar presented findings from the MMRF’s MyDRUG platform trial (Abstract 1931). Unlike traditional clinical trials, which test one drug or a single combination of drugs, the MyDRUG platform trial is a phase 1/2 study of patients with RRMM, who have received at least one prior but no more than 3 prior therapies and were exposed to both a proteasome inhibitor and an immunomodulatory drug and had early relapse after initial treatment or were primary refractory to initial treatment. Through different subprotocols, patients received targeted agents, immunotherapy, or novel agents in combination with a backbone regimen of Ninlaro/Pomalyst/dexamethasone, also known as IPD.
In this abstract, Dr Kumar and colleagues reported data collected from thirty-eight patients who were treated with Darzalex plus IPD. Results showed:
Stay tuned for more updates from ASH 2022!
33 CoMMpass abstracts demonstrate the ongoing value of MMRF data sets to myeloma research
Multi-arm MyDRUG clinical trial reports translational data from cobimetinib arm and initial safety and activity data for daratumumab arm
Norwalk, Conn., December 12, 2022— Today, the Multiple Myeloma Research Foundation (MMRF) announced that new insights related to novel targets, risk assessment, and potential improved treatment approaches generated through its landmark CoMMpass℠ Study and MyDRUG℠ platform trial will be presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition in New Orleans, Louisiana from December 10 – 13, 2022.
Data from the MMRF helped drive findings of 33 studies featured at ASH, with the MMRF CoMMpass Study being cited in nine oral abstracts, highlighting the utility and value of the oft-used study by the global myeloma research community.
“Since its inception, the CoMMpass Study has been used by hundreds of researchers worldwide and continues to promote a level of collaboration that is essential to an increased understanding of myeloma,” said George Mulligan, Ph.D., Chief Scientific Officer of the MMRF. “At the same time, the MyDRUG trial continues to make an impact by assessing the way targeted therapies interact with specific biomarkers found in patients. The insights gained by these two innovative studies demonstrate the importance of generating and sharing a wide array of data.”
MMRF MyDRUG platform trial advances community’s understanding of targeted therapies
Also featured in multiple abstracts at this year’s ASH meeting is MyDRUG, a first-in-myeloma Phase 1/2 platform clinical trial conducted by the MMRF’s Multiple Myeloma Research Consortium (MMRC), a network of leading cancer centers that investigate promising early-stage therapies. The MMRC focuses primarily on trials like MyDRUG that require the collaboration of many sites, given their specificity and scale. MyDRUG builds upon discoveries of common cancer-associated mutations observed in the CoMMpass Study. The goal of MyDRUG is to determine if precision medicine strategies using targeted therapies that are already approved in other cancers are safe and effective in myeloma patients with specific mutations.
Notable findings at this year’s ASH meeting related to the MyDRUG study include:
About the MMRF CoMMpass StudySM
The MMRF CoMMpass Study is an ongoing longitudinal study of patients with newly diagnosed active multiple myeloma. The goal is to map the genomic profile of each patient at diagnosis and each relapse to clinical outcomes in order to develop a more complete understanding of how patients respond to treatments. The MMRF initiated the CoMMpass Study more than ten years ago to address the need for a large, comprehensive, genomic and clinical data set that was publicly available to researchers to realize the potential of personalized medicine. The insights generated by CoMMpass have led to groundbreaking discoveries that have transformed the research community’s understanding of myeloma at a genomic level.
About the MMRF MyDRUG StudySM
The MMRF MyDRUG study is the first platform trial in myeloma. The purpose of MyDRUG is to test targeted therapies not yet approved in myeloma, in combination with a standard of care oral triplet therapy, in functionally high-risk multiple myeloma patients who demonstrate specific genetic alterations.
About the Multiple Myeloma Research Foundation (MMRF)
A pioneer in personalized medicine, the Multiple Myeloma Research Foundation (MMRF) seeks to find a cure for all multiple myeloma patients by relentlessly pursuing innovations that accelerate the development of personalized treatments for cancer. Founded in 1998 by Kathy Giusti, a multiple myeloma patient, and her twin sister Karen Andrews as a 501(c)(3) nonprofit organization, the MMRF has created the benchmark business model around cancer—from data to analytics to the clinic. The MMRF identifies barriers and then finds the solutions to overcome them, bringing in the best partners and aligning incentives in the industry to drive better outcomes for patients. Since its inception, the organization has collected thousands of samples and tissues, opened nearly 100 trials, helped bring more than 15 FDA-approved therapies to market, and built CoMMpass, the single largest genomic dataset in myeloma. Today, the MMRF is building on its legacy in genomics and is expanding into immunotherapy, as the combination of these two fields will be critical to making personalized medicine possible for all patients. The MMRF has raised more than $500 million and directs nearly 90% of the total funds to research and related programs. To learn more, visit www.themmrf.org.
Multiple Myeloma Research Foundation Media Contact:
C.J. Volpe, Director, PR and Communications
203.652.0453
[email protected]
Norwalk, CT, Oct. 25, 2022 — The Janssen Pharmaceutical Companies of Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) approval of TECVAYLI™ (teclistamab-cqyv) for the treatment of adult patients with relapsed or refractory multiple myeloma, who previously received four or more prior lines of therapy, including a proteasome inhibitor, immunomodulatory drug and anti-CD38 monoclonal antibody. TECVAYLI™ is a first-in-class, bispecific T-cell engager antibody that is administered as a subcutaneous treatment.
To read Janssen’s full press release click here.
###
Multiple Myeloma Research Foundation Media Contact:
C.J. Volpe, Director, PR and Communications
203.652.0453
[email protected]
As part of the Multiple Myeloma Research Foundation (MMRF) immune atlas pilot project, we compared immune cells of multiple myeloma bone marrow samples from 18 patients assessed by single-cell RNA sequencing (scRNA-seq), mass cytometry (CyTOF), and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) to understand the concordance of measurements among single-cell techniques. Cell type abundances are relatively consistent across the three approaches, while variations are observed in T cells, macrophages, and monocytes. Concordance and correlation analysis of cell type marker gene expression across different modalities highlighted the importance of choosing cell type marker genes best suited to particular modalities. By integrating data from these three assays, we found International Staging System stage 3 patients exhibited decreased CD4+ T/CD8+ T cells ratio. Moreover, we observed upregulation of RAC2 and PSMB9, in natural killer cells of fast progressors compared with those of nonprogressors, as revealed by both scRNA-seq and CITE-seq RNA measurement. This detailed examination of the immune microenvironment in multiple myeloma using multiple single-cell technologies revealed markers associated with multiple myeloma rapid progression which will be further characterized by the full-scale immune atlas project.
Significance:
scRNA-seq, CyTOF, and CITE-seq are increasingly used for evaluating cellular heterogeneity. Understanding their concordances is of great interest. To date, this study is the most comprehensive examination of the measurement of the immune microenvironment in multiple myeloma using the three techniques. Moreover, we identified markers predicted to be significantly associated with multiple myeloma rapid progression.
The US Food and Drug Administration (FDA) has granted accelerated approval to teclistamab (Tecvayli, Janssen Biotech, Inc.) for adults with relapsed or refractory multiple myeloma after at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.