
Jeff is a husband, father, and grandfather who lives with multiple myeloma every day. Despite his challenges, he faces them with optimism and a smile. Having gone through a long journey with the illness, Jeff shares his experiences, both the highs and lows, with a positive and enthusiastic attitude. His goal is to help others by offering insights from his personal battle with the disease. Jeff has participated in the MMRF Team for Cures Walk/Run Detroit since its inception and his commitment to the mission of accelerating a cure for every myeloma patient is evident in his continued efforts each year. He believes “being a 19-year survivor with a zest for life provides hope for patients” – and we agree!
How did you get involved with the MMRF?
I began to read more and visit the MMRF website after my formal diagnosis of myeloma in January 2018. I had my first solitary isolated plasmacytoma in 2006 and another in 2014. I was so impressed by my oncologist’s involvement in the Moving Mountains for Multiple Myeloma and taking patients on these adventures with him that I recognized how important the MMRF was in researching finding a cure and giving each of us hope for a positive quality of life.

Why did you choose to participate in the MMRF Walk/Run?
When it was announced that Southeast Michigan was going to have its first fundraising walk, I knew I had to participate. This will be my fourth walk. I was out of town for the second walk but my wife and I along with my daughter and son-in-law did a 5k in Cincinnati wearing our shirts. The walk became important as a vehicle to raise more awareness of myeloma, help to generate more funds for continued research, provide support for those who are in treatment, and those of us who are coping with the diagnosis, and provide hope for an opportunity to live our best lives possible post-treatment.
The Spirit of Hope is given to “individuals/groups who inspire hope and show extraordinary commitment to the MMRF.” What does being given the award mean to you?
I never thought about awards, just about fundraising and support for an organization focused exclusively on patients like me to provide continued research and clinical trials to allow patients to have a positive quality of life. And find a way to cure myeloma in the very near future. I am humbled and appreciate that MMRF recognized my contribution to the organization and my commitment to finding a way to help each of us live our best lives.

How have you found perseverance in light of obstacles? Please share any stories that have given you strength. I am a person with a positive outlook and grateful for the love of my family and friends. I have many goals in my life and their support has given me the strength to keep moving forward and appreciate each day.
I have decided to give back as a volunteer with several causes. I am a weekly volunteer at my cancer center, helping patients, nurses, and clinicians with anything they need. I am so grateful to my team for their support in getting me through the difficult times so I pay it forward. I believe being a 19-year survivor with a zest for life provides hope for patients. While they say I am inspiring, I am constantly being inspired by their will and determination to get through their diagnosis. I have met so many wonderful people and feel I was a help when I leave. I work with our social worker with anything she asks. I’ll speak with patients about my experience, listen to their concerns, and try to help in any way possible.
I also volunteer with our community food bank. So many people have food insecurity and sadly there is so much food waste. Twice a week I pick up food and deliver it to a Meals on Wheels location that cooks and sends meals out to those in need.
During the toughest part of my induction therapy, when neuropathy crippled my legs, feet, and hands and I couldn’t walk or grip silverware, I was touched by the family and friends who came from out of town (25 people, some who showed up multiple times) to help me and my wife when I was at my lowest point. Their love and support were a tremendous help in getting me back on my feet as they took me to therapy appointments and helped me do my exercises to get me back to a positive quality of life.
Do you have a favorite mantra, quote, lyric that gives you strength?
At this point in life, my mantra is to “Keep on Truckin’”, and share as many experiences as possible and memories as possible with my family. We enjoy being together and sharing good times. Music plays a big role in my life and experiencing concerts together brings me joy. I’m a huge Grateful Dead fan and we’ve been to several Dead and Company shows together. This spring my wife and family are traveling together to Las Vegas to see shows at the Sphere. We are all so excited!

Anything to add?
I really believe no one knows what tomorrow will bring. Live each day to its fullest. Do my best to be healthy. Do my Mitzvahs (good deeds), slow down, be kind. Show family and friends how special they are in your life. Be grateful and kind. If I practice these traits, I’m living my dream.
—
The MMRF is thrilled to recognize Jeff Levison as the MMRF Spirit of Hope Honoree at the 2025 MMRF Team for Cures: Detroit Walk/Run. Donate to Jeff Levinson and his team, K- Farm Guys, today to accelerate a cure!
This award is presented at every Walk/Run to a patient, caregiver, or family who inspires hope through their resilience, perseverance, and dedication to the MMRF and its mission.
Learn more about the Spirit of Hope award and the 2025 recipients.
We are deeply saddened about the passing of Richard “Dick” Parsons, a prominent and accomplished leader who held top posts at Time Warner and Citigroup among other companies. Dick was a very close friend to and long-time supporter of the Multiple Myeloma Research Foundation.
Diagnosed with multiple myeloma in 2015, Dick soon became a strong advocate for raising awareness of this uncommon blood cancer. Myeloma disproportionately affects the Black community, and Dick was an incredible partner who voiced the need for stronger data and research to drive cures. In fact, Dick participated in the MMRF CoMMpass StudySM, contributing his own data to this large longitudinal study to advance research efforts to benefit all patients. He championed the importance of taking action by learning about the disease and advocating for one’s own care, which remains an invaluable gift and legacy to the myeloma community.
Dick will be greatly missed. We extend our most heartfelt condolences to his family and loved ones.

Richard “Dick” Parsons with MMRF Founder Kathy Giusti and Sid Mukherjee.
In September 2024, Sarclisa (isatuximab) became the first drug to be approved by the FDA for use with Velcade, Revlimid, and dexamethasone (Isa-VRd) for adult patients who are newly diagnosed with multiple myeloma but unable to receive high-dose chemotherapy and a stem cell transplant commonly used in myeloma treatment.
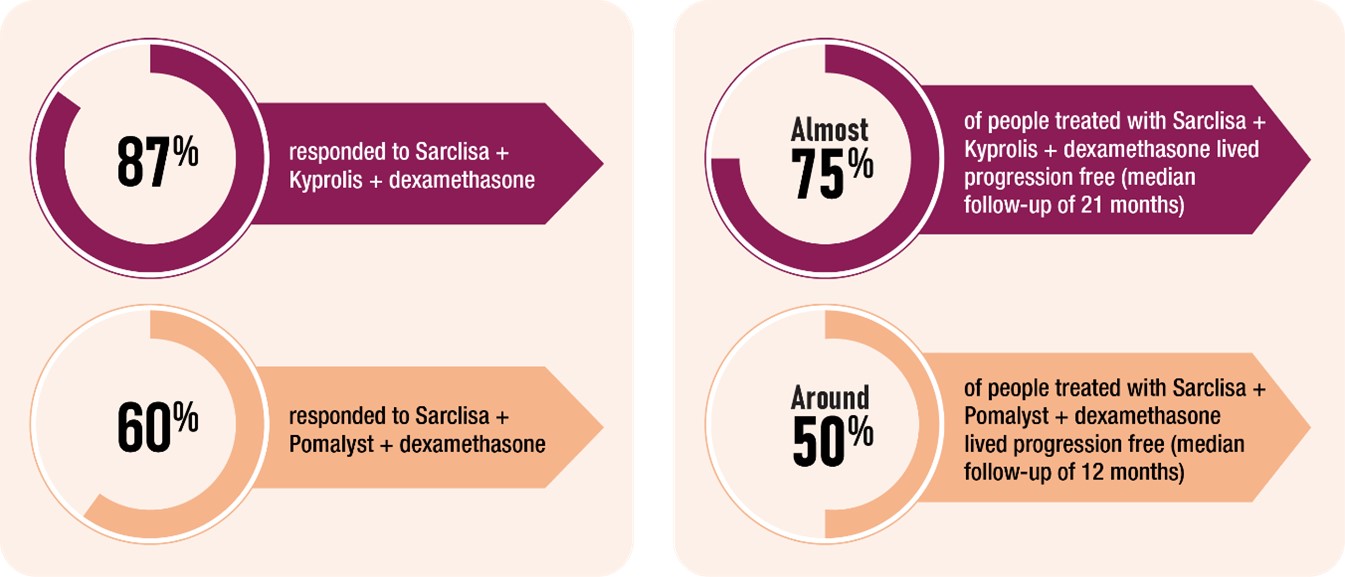
With this new approval, patients with newly diagnosed myeloma have an exciting new treatment option. Previously, Sarclisa had been approved for use as myeloma treatment, in combination with Pomalyst or Kyprolis and dexamethasone, in later lines of therapy.
In clinical trials, most patients who received a combination therapy that included Sarclisa responded to the treatment, and many lived without their myeloma getting worse.
Sarclisa is a monoclonal antibody, a type of immunotherapy agent that is designed to work with your immune system to find, bind to, and destroy myeloma cells in your body.
Importantly, Sarclisa is one of a class of agents that is able to target a specific component of myeloma cells, a protein called CD38. Myeloma treatments that target CD38, which include Darzalex (daratumumab), have several advantages, including the potential for deep responses and longer periods in which myeloma does not progress.
Patients are given Sarclisa by intravenous infusion. Treatment spans cycles of 28 days, in which Sarclisa is administered along with the other agents in the combination therapy. In the first cycle, Sarclisa is usually given weekly. For the remainder of treatment, Sarclisa is usually given every 2 weeks. Currently, studies are looking at whether giving Sarclisa as an injection (instead of an infusion) is an option.
As with most myeloma treatments, there are side effect risks with Sarclisa. Infusion reactions may occur during or shortly after the medication is given. These reactions are most common during the first infusion and can produce fever, chills, nausea, headache, difficulty breathing, low blood pressure, and flushing.
Patients receive medicines before each Sarclisa infusion to help make infusion reactions less frequent and severe. While receiving Sarclisa, patients are monitored for infusion reactions. If a reaction occurs, the infusion can be slowed down or stopped depending on the severity of the reaction.
Low white blood cell, low red blood cell, and low platelet counts occur in most patients. These effects can often be reversed by adjusting the treatment or with supportive measures like transfusions or medications to boost blood cell production. Other side effects include diarrhea, fatigue and nausea, which are typically mild and temporary. Respiratory infections, such as colds or coughs, may develop and usually improve with treatment.
Interested in learning more about Sarclisa? Talk to an MMRF patient navigator at the Patient Navigation Center at 1.888.841.6673 (Monday through Friday, 9:00 am to 7:00 pm ET) or email patientnavigator@themmrf.org.
Additional Resources
MMRF Patient Toolkit: Multiple Myeloma Treatment Overview
All our downloadable resources: Education Resource Hub
The MMRF aims to incorporate a variety of new bispecific antibodies, cell therapies, checkpoint inhibitors and novel small molecules into the Horizon study.
This article presents a Q&A with MMRF Patient Navigation Center nurses Grace Allison and Brittany Hartmann on key takeaways from the 2024 annual meetings of the National Comprehensive Cancer Network (NCCN) and the Journal of the Advanced Practitioner in Oncology (JADPRO) on recent multiple myeloma treatment advances.
Low white blood cell, red blood cell, and platelet counts commonly occur with many different myeloma therapies, and they are seen even more frequently in patients receiving CAR T-cell therapy.
Because low white blood cell counts increase the risk of infections, medications called growth factors (for example, filgrastim) may be given to patients who receive CAR T-cell therapy. These drugs help the body produce white blood cells. Low red blood cell counts can cause fatigue and shortness of breath and are usually treated with blood transfusions or drugs (for example, erythropoiesis-stimulating agents) to help the body make more red blood cells. For low platelets, which raise the risk of bleeding and bruising, platelet transfusions may be necessary in severe cases.
Infections are also a common side effect of CAR T-cell therapy. Before starting treatment, patients are usually given medications to prevent infections—for example, acyclovir to prevent viral infections. Antibiotics and antifungals are commonly given, as well. Intravenous immunoglobulin (IVIG), which boosts a patient’s immune system following CAR T-cell therapy, is also often given. As another strategy for infection prevention, many treatment centers are re-vaccinating patients following CAR T-cell therapy.
If a fever develops after CAR T-cell therapy, even if it’s just low grade, patients should immediately report it to their care teams. The doctor will investigate to see if it’s caused by an infection. This may require blood tests and possibly a chest x-ray to rule out the possibility of an active infection.
If there’s no sign of infection, the doctor will see if the fever is caused by a reaction called cytokine release syndrome or CRS. In CRS, the body’s immune system gets revved up too high, resulting in an overreaction that can cause fever, chills, fatigue, muscle aches, nausea, and a fast heartbeat. In more serious cases, CRS can cause problems with the lungs, liver, or heart. Fortunately, CRS is a condition that the care team will be ready to address. Treatments for CRS include tocilizumab and steroids.
Side effects that are of particular concern are lesser-known neurotoxicities, including a condition called immune effector cell-associated neurotoxicity syndrome—better known as ICANS. In ICANS, inflammation interferes with the brain, leading to symptoms like confusion, difficulty speaking, headaches, and feeling very sleepy. In more serious cases, seizures or changes in alertness can occur.
ICANS typically occurs within a few days of receiving CAR T-cell therapy. The duration varies, but the condition usually resolves within 1 to 2 weeks with appropriate management. To manage ICANS and other neurotoxicities, the care team will usually consult with a neurologist. Management strategies can include using anti-seizure medications.
Another concern is more serious Parkinsonian-like syndromes, which are conditions that cause symptoms like those seen in Parkinson’s disease, such as stiffness, tremors, and balance problems. In studies, some patients who received the CAR T-cell agent Carvykti developed Parkinsonian-like syndromes 40 to 60 days after treatment. The symptoms may persist, requiring long-term management and supportive care.
As with CAR T-cell therapy, CRS and ICANS can develop in patients receiving bispecific antibody therapy. CRS is more common than ICANS, but the risk for both is much lower with bispecifics than with CAR T. When these conditions develop, it’s usually at the beginning of treatment, during the step-up doses. This is why these doses are given in a hospital or other treatment facility. After all the step-up doses have been completed, the risk of CRS and ICANS is lower.
Bispecific antibodies can weaken the immune system, increasing the risk of infections. Patients and caregivers should be vigilant for signs of infection such as fever, chills, or a persistent cough. Maintaining open communication with your healthcare team about any symptom is important.
The bispecific antibody Talvey has some unique side effects. Talvey targets a protein called GPRC5D, which is present on myeloma cells. It’s also present on other cells in the body, including those found in the mouth, skin, and nails. Because of this, Talvey can cause side effects in these parts of the body, such as a full-body rash, peeling of the skin, and changes in the fingernails. Though inconvenient and unpleasant, these side effects rarely create difficulty with daily activities and instead tend to be quality-of-life issues.
Oral side effects can include taste changes and mouth sores. Difficulty swallowing is another possible side effect and can be concerning, because it can cause lack of appetite, which can lead to weight loss. Proper nutrition is important not just to maintain day-to-day health but especially to help fight off myeloma and the side effects of myeloma treatment.
Care teams often recommend a thick barrier lotion like Aquaphor for rashes and peeling. Other products are available to help strengthen and protect the nails. For oral side effects, special mouthwashes (for example, dexamethasone) can be used for dry mouth.
Consulting a nutritionist early on is important, as a nutritionist can recommend strategies for coping with mouth problems such as mouth sores, changes in sense of taste, dry mouth, and difficulty swallowing before they become a serious issue that interferes with eating.
For patients with comorbidities, early referral to a nutritionist is important. Additionally, physical therapy for strength maintenance and muscle protection can help patients maintain their quality of life.
One presentation at NCCN noted that a strategy for balancing comorbidity management and myeloma treatment is a drug holiday—that is, a period where a patient does not take any medication. This is particularly an option when the myeloma is well controlled. This strategy can make patients nervous that the myeloma will come roaring back. Care teams, however, are very much on board with drug holidays when appropriate, and they don’t fear that it will affect long-term outcomes.
CELMoDs, a new class of myeloma drug, work like immunomodulators such as Revlimid and Pomalyst and are orally administered. They help the immune system recognize and attack myeloma cells, and they kill myeloma cells directly. CELMoDs have been shown to be effective even in patients whose myeloma has become resistant to some treatments.
One point that came up at JADPRO was the importance of making CELMoDs and oral agents more widely available—for instance, to patients being treated in community facilities. Expanding the availability of these agents would make these treatments accessible to patients who do not live near specialized treatment facilities and could also reduce or eliminate the need for patients to spend time receiving infusions. Overcoming these barriers offers the potential to improve patient quality of life while also providing treatments that have shown to have great responses.
We know that the percentage of myeloma deaths is higher in Black, Latinx, and LGBTQ+ patients, largely because of unequal access to care and lower rates of early detection and screening. The fact that there is an awareness of this discrepancy, and that it’s being discussed, is an important step forward. This is an area that the MMRF is actively addressing by raising awareness among and providing education to care providers.
Other efforts to improve care equity have focused on making participation in clinical trials easier for a broader population of patients. Strategies like offering travel assistance and expense reimbursement, and opening trials in smaller community settings and not just large academic hospitals, allow patients from rural areas to participate.
Finally, helping patients manage treatment-related costs is another area that has received greater attention. This can involve helping patients navigate the ever-changing landscape of insurance or offering programs that provide financial support. Receiving CAR T-cell therapy involves a significant commitment and can disrupt daily life, including work responsibilities. At present, this treatment requires travel to a specialized medical center, which for many patients will be far from home, adding time and logistical challenges. This time away from work and daily life can be a burden, both emotionally and financially. Several organizations have developed programs to provide financial support for treatment-related expenses, such as the cost of medication, travel to a treatment center, and hotels.
HOUSTON & SEATTLE, December 17, 2024–Indapta Therapeutics, Inc., a privately held clinical stage biotechnology company developing next-generation cell therapies for the treatment of cancer and autoimmune diseases, announced today it has closed a $22.5 million round of new financing to accelerate the clinical development of its differentiated allogeneic Natural Killer (NK) cell therapy. Current investors RA Capital Management, LP, Leaps by Bayer, the impact investment arm of Bayer AG, Vertex Ventures HC, Pontifax, and the Myeloma Investment Fund, the venture philanthropy subsidiary of the Multiple Myeloma Research Foundation, completed the round.
“This funding will enable us to generate significant additional data in our ongoing trial of IDP-023 in cancer as well as initial data from our first trial in autoimmune disease,” said Mark Frohlich, Indapta’s CEO. “Preliminary results of IDP-023 in cancer are encouraging and we look forward to initiating our Phase 1 trial for multiple sclerosis in Q1 2025. This financing, together with our recently announced collaboration with Sanofi, highlights the promise of our differentiated platform.”
Read the full press release here.
Welcome to our final recap of the latest research on myeloma treatments presented at the American Society of Hematology (ASH) Annual Meeting in San Diego. Highlights from the final day included:
Let’s jump into the latest findings!
Researchers from Greece demonstrated that treatment with Darzalex can significantly delay the progression from smoldering multiple myeloma (SMM) to active disease in high-risk patients. Data from this abstract, simultaneously published in the New England Journal of Medicine (Dimopoulos et al.), showed that patients receiving Darzalex had a 51% lower risk of progression (that is the time until myeloma is detected) compared to those under active monitoring, the current standard of care after a median of more than 5 years of follow-up. The most common serious side effect in both arms was hypertension, affecting approximately 5%. Approximately 5% of patients in the Darzalex arm discontinued treatment.
High-risk SMM is an asymptomatic precursor to multiple myeloma, and early treatment strategies may improve outcomes for patients with a high risk of progression to active disease. The researchers emphasized that findings from this phase 3 trial specifically apply to patients with high-risk SMM. Future research will explore whether Darzalex alone or in combination with other medications could help prevent disease progression in patients with high-risk SMM.
Two abstracts showed that Sarclisa in combination with the current standard of care demonstrated significant treatment benefits in patients with newly diagnosed multiple myeloma (NDMM). Sarclisa is an anti-CD38 antibody in the same class as Darzalex and is administered intravenously.
The first abstract showed that Sarclisa in combination with Revlimid (lenalidomide), Velcade (bortezomib), and dexamethasone (Isa-VRd) induction therapy resulted in a 30% reduction in the risk of disease progression in transplant-eligible NDMM patients compared to VRd. Half of patients treated with Isa-VRd achieved minimal residual disease (MRD) negativity, indicating no detectable cancer cells, after 18 weeks of treatment. Side effects such as infections, diarrhea, and peripheral neuropathy were consistent with previous clinical trial findings of Isa-VRd.
In the next abstract, Dr. Orlowski and colleagues from MD Anderson Cancer Center reported that Isa-VRd induction therapy followed by maintenance with Isa-Rd demonstrated higher and sustained MRD negativity rates compared with the current standard of care in NDMM patients not eligible for a transplant. This analysis of the phase 3 study included 446 patients. Side effects of Sarclisa observed in this study were consistent with prior studies of Sarclisa and VRd.
Researchers conclude that Sarclisa-based quadruplets may represent an alternative new standard of care for patients with NDMM.
For patients with relapsed or refractory multiple myeloma considering treatment with Abecma (idecabtagene vicleucel), a CAR T-cell therapy, understanding factors that influence the effectiveness and side effects is important. Currently it is available for patients who have had two or more prior lines of therapy.
In a study of 108 patients, Dr. Hansen and researchers from the H. Lee Moffitt Cancer Center found that patients with high disease burden, such as advanced disease stage, and the presence of biomarkers of inflammation like ferritin and C-reactive protein before starting Abecma treatment were more likely to develop side effects such as neurotoxicity (ICANS). Patients who had extramedullary disease (ie, the presence of myeloma cells outside of the bone marrow) and those who had received prior treatment with a BCMA-targeting agent were less likely to have durable responses.
These findings highlight the importance of pre-treatment factors in shaping outcomes with ide-cel therapy and may help guide personalized approaches to optimize benefits while managing risks.
In the next abstract, researchers in the United Kingdom reported that treatment with Carvykti led to significantly higher rates of MRD-negativity in patients with RRMM who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), compared to standard therapies of Pomalyst (pomalidomide), Velcade, and dexamethasone (PVd) or Darzalex, Pomalyst, and dexamethasone (DPd). Carvykti is currently available for patients who have had one or more prior lines of therapy. These findings further support the use of Carvykti patients with Revlimid-refractory MM.
Dr. Dima and researchers at the Cleveland Clinic Taussig Cancer Institute looked at how effective Tecvayli, a BCMA-targeting bispecific antibody, is for patients with relapsed/refractory multiple myeloma (RRMM) who previously received other BCMA-directed therapies like CAR T-cell therapy or antibody-drug conjugates (ADC). In this abstract, researchers found that while Tecvayli is effective for these patients, outcomes were generally better for those who hadn’t received prior BCMA treatments. Currently, it is available for patients who have had four or more lines of therapy.
Patients previously treated with BCMA-directed therapy had slightly lower response rates to and shorter median progression-free survival (PFS, time before the disease worsened) compared to for those without prior BCMA-directed therapies. The study also highlighted the importance of timing between BCMA-directed therapies. Patients who waited more than 9 months after their last BCMA-directed therapy before starting Tecvayli had better outcomes. Side effects like CRS and neurotoxicity (ICANS) were similar regardless of prior BCMA therapy; however, patients with prior BCMA-directed therapy were more likely to experience low platelet counts early in treatment. These findings suggest that Tecvayli is a valuable option for patients previously treated with BCMA therapies, and patients and caregivers should discuss the timing of starting Tecvayli with their healthcare team.
Dr. Tomasson and colleagues from the University of Iowa reported that Elrexfio, a bispecific antibody targeting BCMA, in combination with Kyprolis (carfilzomib) and dexamethasone shows promise for treating patients with RRMM. Currently, Elrexfio is available for patients who have had four or more lines of therapy.
In this abstract, researchers data from an early-phase study involving 12 patients, all of whom had received at least one or more prior lines of therapy. The most common side effects included fatigue, mild cytokine release syndrome (CRS), and low blood counts. Further studies ongoing will evaluate the benefits of Elrexfio combined with Kryprolis and dexamethasone in a larger population.
Researchers from Brazil reported that belantamab mafodotin combined with Velcade and dexamethasone (BVd) shows significant overall survival benefit, reducing the risk of death by 42% in multiple myeloma at or after first relapse compared to Darzalex with Velcade and dexamethasone (DVd). In this abstract, data was analyzed from 494 patients, of which 51% of patients had received 1 previous line of therapy, 52% had received prior Revlimid, while 34% of patients no longer responded to Revlimid; 28% had high-risk chromosomal abnormalities. The researchers note that additional studies will shed light on if BVd may be a potential new standard of care in MM at first relapse or later.
Cevostamab is an investigational bispecific antibody that targets FcRH5, a new target on myeloma cells. In this study of 167 heavily pretreated patients (between 2 and 18, with a median of 6), Dr. Richter and researchers at Mount Sinai found that cevostamab demonstrated good response rates. Patients who had not previously received BCMA-targeted therapies responded better than those who had. Almost all patients (96%) were resistant to all three standard treatment classes: proteasome inhibitors, immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies. A majority (57%) of patients had received one or more prior BCMA-targeted therapy. Common side effects included CRS, low white blood cell counts, and fatigue. Further analysis will evaluate the use of cevostamab in larger trials.
CELMoDs are a new class of oral myeloma drugs that work like immunomodulators such as Revlimid and Pomalyst. They also stimulate the immune system and kill myeloma cells directly, even for myeloma that has become resistant to certain treatment. Researchers from Canada presented their findings from an early phase trial evaluating mezigdomide, an oral CELMoD, in combination with dexamethasone and either Velcade (Mezi-Vd) or Kyprolis (Mezi-Kd). In this study, 77 patients who have had one or more prior lines of therapy received Mezi-Vd and 27 patients received Mezi-Kd. The authors reported that Mezi-Vd and Mezi-Kd demonstrated high response rates and side effects such as low white blood cell counts, infections, and low platelet counts.
Researchers from Israel reported data from an early phase clinical study of HBI0101, an anti-BCMA CAR T-cell therapy. Their study included 84 patients, most of whom were triple class refractory, showed high response rates and MRD negativity rates in patients treated with HBI0101. Common side effects included CRS, low white blood cell, low red blood cell, and low platelet counts.
Dr. Freeman and researchers at the H. Lee Moffitt Cancer Center reported their findings from an early phase trial evaluating anitocabtagene autoleucel, another anti-BCMA CAR T-cell therapy. The trial included 58 patients with RRMM who have received at least three prior lines of treatment. Patients treated with anitocabtagene autoleucel achieved high response rates and MRD negativity rates. Common side effects observed were CRS, low white blood cell, low red blood cell, and low platelet counts.
Further analysis will evaluate the use of these new therapies in larger trials.
We hope you enjoyed our daily recap of the latest advances in myeloma treatment. Keep an eye on themmrf.org for future updates!
Welcome to the second day of our recap of the latest findings on myeloma treatments reported at the American Society of Hematology (ASH) meeting in San Diego.
Highlights from today included:
Let’s take a closer look!
Several abstracts highlighted promising results on the use of Tecvayli (teclistamab) in combination with other drugs for patients with newly diagnosed multiple myeloma (NDMM) and those who relapse after one or more line of treatment. Currently, it is available for patients who have had four or more lines of therapy.
In the first abstract, 49 patients with transplant-eligible NDMM were treated with Tecvayli in combination with Darzalex, Revlimid, and dexamethasone with or without Velcade (Tec-DRd) or (Tec-DVRd) as induction therapy. Researchers from Germany reported that 35 of 36 patients who completed at least 3 treatment cycles achieved minimal residual disease (MRD) negativity, indicating no detectable cancer cells. The most common side effects were infections and cytokine release syndrome (CRS), which presents as flu-like symptoms and is a common side effect of the bispecific antibodies. Further analysis will evaluate these combinations in larger trials.
Revlimid maintenance therapy after autologous stem cell transplantation (ASCT) has been the standard-of-care for transplant-eligible NDMM. However, patients eventually relapse, supporting the need for better strategies for maintenance therapy. In this abstract, researchers from Italy reported data from an early phase trial that Tecvayli with or without Revlimid could be safely given as maintenance therapy for 90 patients with transplant-eligible NDMM. Common side effects included infections, low white blood cell counts, CRS, cough, diarrhea, and fatigue. All patients enrolled in the study who received Tecvayli with or without Revlimid as maintenance therapy achieved MRD negativity. These findings support the ongoing study of Tecvayli alone or in combination with Revlimid as maintenance therapy.
In the next abstract, Dr. D’Souza and colleagues at the Medical College of Wisconsin showed that Tecvayli in combination with Darzalex and Pomalyst may lead to better responses, especially in patients with relapsed or refractory multiple myeloma (RRMM) who had one or more lines of therapy. Common side effects reported in this early phase trial of 27 patients with RRMM were infections, low white blood cell, red blood cell, and low platelet counts. The researchers concluded that, pending the results of a larger study, Tecvayli in combination with Darzalex and Pomalyst may be a new triplet therapy for early relapsed myeloma.
Etentamig (formerly ABBV-383) is a new bispecific antibody that targets B-cell maturation antigen (BCMA) that is being evaluated with monthly dosing from the beginning of treatment, unlike Tecvali and Elrexfio which are administered weekly through 24 weeks after initial step-up.
Dr. Rodriguez and colleagues at Mount Sinai presented results from an early phase trial of ABBV-383 combined with Darzalx and dexamethasone in this abstract. The study, which aimed to identify the best dosage of ABBV-383, included 60 patients who received 3 or more prior lines of therapy including a proteasome inhibitor and an immunomodulatory drug. Of this group 70% had received anti-CD38 mAb therapy. Most common side effects included low platelet, white blood cell, and red blood cell counts, CRS and fatigue. Their findings suggest that ABBV-383 in combination with Darzalx and dexamethasone is tolerable and early response rates were promising. Ongoing trials will offer more information on the potential use of ABBV-383 in RRMM.
In this abstract, Dr. Usmani and colleagues from Memorial Sloan Kettering Cancer Center showed belantamab mafodotin combined with VRd led to good response rates in patients with NDMM who are ineligible for transplant. Vision problems were the most common side effect. These side effects were managed by briefly stopping treatment or reducing the dose of belantamab mafodotin, allowing most patients to continue treatment. With these promising results, future studies will continue to evaluate the potential of belantamab mafodotin in patients with newly diagnosed disease.
In the final abstract, Dr. Foster colleagues at the University of Virginia reported that Darzalex in combination with Revlimid maintenance therapy resulted in better remission rates (MRD-negative) and longer disease control compared to Revlimid alone. The researchers evaluated data from the phase 3 AURIGA study that included 200 transplant-eligible NDMM patients who were MRD-positive after ASCT regardless of age, race, or risk profile. Findings from this trial revealed that maintenance therapy with Darzalex combined with Revlimid prolonged the time to disease progression across all subgroups of patients, including those with high-risk disease. Low white blood cell counts and infections were the most common side effects observed in this phase 3 trial of 200 patients with NDMM.
The researchers conclude that these results further support adding Darzalex to Revlimid maintenance therapy for patients with NDMM following stem cell transplant.
Be sure to come back tomorrow for our final day of updates in the treatment of myeloma at ASH 2024.
Welcome to our 2024 recap of the latest research on myeloma treatments reported at the American Society of Hematology (ASH) meeting that kicked off Saturday in San Diego. Highlights from today included:
Let’s dive in!
Autologous stem cell transplantation (ASCT) followed by Revlimid (lenalidomide) maintenance continues to be the standard of care for eligible patients with newly diagnosed multiple myeloma (NDMM). Currently, patients with myeloma typically stop maintenance therapy when their disease progresses, meaning there is no set timeframe to stop treatment. However, researchers are finding that patients with sustained (durable) minimal residual disease (MRD) negativity (that means that no myeloma cells were detected) may be able to safely stop maintenance therapy.
In this abstract, researchers from Greece found that maintenance therapy could be stopped for certain patients might be able to stop taking Revlimid maintenance therapy if they were consistently MRD-negative for 3 years. The study included 194 patients who received induction with proteasome inhibitor-based regimens (ie, regiments with Velcade or Kyprolis) and underwent ASCT.
The researchers conclude that sustained MRD negativity after ASCT and a completion of 3 years of Revlimid maintenance may guide the safe discontinuation of maintenance, but more study in clinical trials is needed.
In an analysis of data collected from the MMRF CoMMpass study, Dr. Shan and researchers at the Albert Einstein College of Medicine showed that those who relapse within 12 months of initial therapy have an overall survival (OS) of less than 24 months, regardless of the presence of high-risk features at diagnosis. Out of the 228 myeloma patients classified as functional high-risk myeloma, 63 did not have high-risk chromosomal features specifically–t(4;14), t(14;16), t(14;20), 1q amplification (amp1q), 17p deletion(del17p), or a p53 mutations. The researchers conclude that future studies are needed to confirm if functional high-risk status could be a useful tool for identifying patients who lack chromosomal changes with poor prognosis.
24-hour urine assessments are a key component of International Myeloma Working Group (IMWG) response criteria for myeloma and are requested in clinical trials even if not routinely used in real-world practice. Recent research has suggested a limited role for 24-hour urine assessment if serum immunofixation or free light chain testing is abnormal. In this abstract, Dr. Banerjee and colleagues at the University of Washington analyzed data from 636 patients in the phase 3 STaMINA trial. Outside of situations such as AL amyloidosis where 24-hour urine assessments remain critical or for patients with urine as their only measurable MM biomarker, our results support the removal of 24-hour urine assessments from future iterations of IMWG response criteria.
While there is no cure, the prognosis for myeloma patients has significantly improved, thanks to advances in treatments over the past 10-20 years. The life expectancy for multiple myeloma patients depends on several factors, including the stage of the disease, genetic factors, age, and overall health. One study followed patients for at least 15 years to look at patient- and disease-related factors that may identify those who have a better chance of living 15 years after their diagnosis.
Findings from one abstract of 323 patients in this study, 40 survived at least 15 years. What factors helped predict long-term survival?
Finally, the first day of ASH revealed some promising results on the use of iberdomide in the treatment of newly diagnosed patients who are not eligible for a transplant and those with intermediate and high-risk smoldering multiple myeloma (SMM), an early stage of myeloma that hasn’t yet caused symptoms but is identified by the presence of a serum monoclonal (M) protein of ≥3 g/dL and/or 10% to 60% clonal bone marrow plasma cells.
Iberdomide is a novel oral cereblon E3 ligase modulator (CELMoD™), which is like but more potent than Revlimid, with a dual function: activate the immune system and directly kill myeloma cells.
The first study, which included 75 patients enrolled in an early phase clinical trial, showed the addition of iberdomide combined with Darzalex (daratumumab) and dexamethasone was very effective, with 30 patients achieving MRD negativity.
The second abstract reported findings from an early phase trial that looked at the use of iberdomide alone or in combination with dexamethasone for the treatment of intermediate- or high-risk SMM). Of the 20 patients examined, Dr. Joseph and colleagues from Emory University found that 79% of those with intermediate- or high-risk SMM responded to iberdomide alone or in combination with dexamethasone. Common side effects included headache, abdominal cramping, dyspepsia, and insomnia.
Further studies will continue to evaluate iberdomide for the treatment of early-stages of disease.
Stay tuned tomorrow for additional updates from trials evaluating treatment of patients with newly diagnosed and relapsed/refractory disease.
Norwalk, Conn., December 6, 2024 – The Multiple Myeloma Research Foundation (MMRF®) today announced upcoming poster and oral presentations at the 66th American Society of Hematology (ASH) Annual Meeting and Exposition, taking place December 7-10 in San Diego, CA. 36 presentations will feature data from the CoMMpassSM and Immune Atlas programs, further solidifying CoMMpass as the reference dataset for advancing multiple myeloma research. The findings shed light on the biology of high-risk multiple myeloma and the tumor immune microenvironment, driving novel insights into disease stratification and treatment strategies.
“Our mission is to deliver a cure for every myeloma patient, and we know that comprehensive, scalable research initiatives are the key to achieving that goal,” said Michael Andreini, President and CEO of the MMRF. “With CoMMpass, we have generated critical insights on distinct subtypes and high-risk genetic markers of multiple myeloma, which has translated to clinical trials and research to inform efforts that can optimize treatments for all myeloma patients.”
Data from CoMMpass and the Immune Atlas programs to be highlighted at ASH include insights demonstrating the unique tumor biology and immune microenvironment of high-risk patients, including:
“CoMMpass has been a cornerstone in advancing our understanding of the complex biology of multiple myeloma for over a decade,” said Jonathan Keats, Ph.D., Assistant Professor and Director of Bioinformatics and the Collaborative Sequencing Center at TGen, as well as a senior researcher involved in CoMMpass. “Through this program, we’ve made significant strides in understanding high-risk multiple myeloma, which is associated with poorer outcomes, and are paving the way for the development of more precise and effective therapies.”
About Multiple Myeloma
Multiple myeloma is a cancer of the plasma cells that develops in bone marrow. It is the second most common blood cancer in the U.S., with 35,750 new cases and 12,590 deaths estimated to occur this year. New agents and therapies have resulted in better outcomes, but most multiple myeloma patients eventually relapse.
About CoMMpassSM
The MMRF CoMMpass study is a collaboration with clinical centers and patients with active multiple myeloma. It is one of the largest and most impactful research efforts in multiple myeloma, following over 1,100 patients across 76 centers for at least eight years. The study maps patients’ tumor genomic profile to clinical outcomes with the goal of developing a more complete understanding of both disease biology and the patient’s response to treatments. With its inclusion in more than 200 published or presented studies, CoMMpass represents the largest longitudinal genomic dataset in multiple myeloma and has led to groundbreaking discoveries that have transformed how researchers understand the biology of the disease. The MMRF continues to support the use of this resource and makes the CoMMpass data available to other researchers globally.
About the Multiple Myeloma Research Foundation (MMRF)
The Multiple Myeloma Research Foundation (MMRF) is the largest nonprofit in the world solely focused on accelerating a cure for each and every multiple myeloma patient. We drive the development and delivery of next-generation therapies, leverage data to identify optimal and more personalized treatment approaches, and empower myeloma patients and the broader community with information and resources to extend their lives. Central to our mission is our commitment to advancing health equity so that all myeloma patients can benefit from the scientific and clinical advances we pursue. Since our inception, the MMRF has raised over $600 million for research, opened nearly 100 clinical trials, and helped bring 15+ FDA-approved therapies to market, which have tripled the life expectancy of myeloma patients. To learn more, visit www.themmrf.org.
Media Contact:
Adam Silverstein
Scient PR
adam@scientpr.com