Updated 7/25/2025
Blenrep (belantamab mafodotin) has now been approved for use in both the European Union and Canada—clear signals that international health authorities recognize its clinical value and potential to improve access to care for patients with relapsed/refractory multiple myeloma.
In the U.S., the FDA has extended its review process with a new target date set for October 23, 2025. This means the agency needs more time to complete its evaluation of Blenrep.
While this delay may leave U.S. patients feeling frustrated or discouraged, it’s important to remember that continued advocacy and real-world stories have a powerful impact. We encourage patients and caregivers to keep the wave of support growing. This collective momentum can help drive awareness, urgency, and ultimately, progress.
The MMRF remains hopeful and continues to support the approval of Blenrep in the U.S., as we believe it offers meaningful clinical benefits and expands access for patients who may not be able to receive other therapies. Here’s why the MMRF sees Blenrep as a valuable and potentially transformative option for the myeloma community.
_____________________________________________________________________________________________
Blenrep specifically targets BCMA, a protein found on myeloma cells, and delivers chemotherapy directly to the cells. One of the most promising aspects of Blenrep is its effectiveness in clinical trials, especially for patients who had only one prior line of therapy. When combined with Velcade (bortezomib) and dexamethasone, Blenrep helped patients live longer without their disease progressing. It also increased overall survival.
Another key benefit? Access.
Unlike CAR T-cell therapy, which is also effective but harder to get, Blenrep is “off the shelf.” It is administered through a 30-minute, IV infusion that can be given in community oncology centers without the need to travel to a major academic hospital. For many, this could be a big step forward in health equity, especially for patients in rural or underserved areas.
Two Big Questions, Two Tough Calls
In a recent meeting, the FDA’s Oncology Drug Advisory Committee (ODAC) reviewed two new combinations involving Blenrep:
1. Blenrep + Velcade + dexamethasone (BVd)
2. Blenrep + Pomalyst (pomalidomide) + dexamethasone (BPd)
The ODAC was asked whether the benefits of these combinations outweigh the risks for patients who have already had at least one prior treatment. After reviewing the evidence, the committee voted 5 to 3 against the BVd combination and 7 to 1 against the BPd combination.
Does Dosing Make a Difference?
We know from earlier studies that Blenrep can cause eye-related side effects. But during the ODAC meeting, the biggest concern was the dose of Blenrep used in the most recent clinical trials. Many experts felt the dose was higher than necessary.
This challenge isn’t unique to Blenrep. In cancer treatment, there’s often a difficult balance between giving enough medication to fight the disease and keeping side effects under control. Sometimes, by the time a drug is ready for approval, the dose may not be fully optimized yet, but the benefits are strong enough to justify moving forward. That’s where programs like the MMRF’s Horizon platform come in. The MMRF is working to optimize dosing strategies for promising therapies like Blenrep—especially in patient groups that have often been left out of trials in myeloma, such as older adults and African Americans. These efforts help ensure the treatments are both effective and tolerable for a broader range of patients. With programs like Horizon in place to continue optimizing care, there’s even more reason to consider Blenrep a valuable option for the myeloma community.
The Patient Voice for Blenrep
In addition to Anne Quinn Young, Chief Mission Officer at the MMRF, as well as leaders from Healthtree and the IMF, several patients who are currently being treated with Blenrep spoke during the open testimony portion of the meeting. Uniformly, each offered stories of hope and gratitude, with most sharing that they had few or no other treatment options. Alternatives were often unavailable, not covered by insurance, or came with side effects they found more difficult to manage.
Patients appreciated that Blenrep was effective for them and that the treatment was easy to receive, requiring only a short 30-minute infusion. For many, the vision-related side effects were manageable with the use of eye drops, sunglasses, or reading glasses—tools they felt were a reasonable trade-off for the side effects experienced.
The Clinician’s Case for Blenrep
Doctors also acknowledged that eye-related side effects are real, but they stressed that these issues are predictable, reversible, and manageable. With regular check-ins and teamwork between oncologists and eye doctors, many patients can stay on treatment safely. Some providers also discussed how adjusting doses can reduce side effects without affecting how well the drug works. With these strategies and the right support, many doctors believe Blenrep could still be a valuable option for patients who need it.
What Happens Next and What this Means for You
While ODAC’s vote is influential, it is not the final decision. While the MMRF supports the FDA approval of Blenrep, the ODAC discussion sends an important message to the myeloma community regardless of approval status: It’s more important than ever to have open conversations with your care team about what treatments are available, what risks they carry, and how they fit into your specific situation.
Here are a few tips to help guide those conversations around shared-decision making:
· Ask your doctor what side effects you might expect and how to manage them.
· Talk about your treatment goals.
· Bring up your lifestyle and daily routines. Let your care team know what matters most to you.
· Ask if there are other treatment options through clinical trials.
Knowing what’s realistic and within reach can help shape next steps. Remember: You don’t have to make these decisions alone. The MMRF Patient Navigation Center is here to support you with one-on-one guidance, resources, and information tailored to your needs.
If you’ve been diagnosed with smoldering multiple myeloma (SMM)—especially if you’ve been told you’re high-risk—you’ve likely wondered: Should I treat my smoldering disease right now, or do I need to wait until it’s active myeloma?
This question was at the heart of a recent meeting held by the Oncologic Drugs Advisory Committee (ODAC) on May 20. ODAC is a group of experts, most of whom are oncologists, who help the FDA decide whether new cancer treatments should receive FDA approval.
At this meeting, ODAC reviewed whether Darzalex Faspro® (a version of daratumumab given as a quick shot under the skin) should be approved as the first treatment for people with high-risk smoldering myeloma.
During detailed discussions of a key clinical trial (the AQUILA study), the committee heard from clinicians, researchers, and advocates representing the FDA, Johnson & Johnson (the manufacturer of Darzalex Faspro), and the myeloma patient population on whether this new use of Darzalex could be beneficial for patients. Two very important questions drove the conversation:
- Can a treatment like Darzalex Faspro help delay progression from smoldering myeloma to active myeloma in high-risk patients?
- If so, does this outweigh any risks that come with the treatment?
Findings from the AQUILA Study
AQUILA compared two groups of people diagnosed as high-risk SMM: One group received Darzalex Faspro early. The other group was monitored closely but without myeloma treatment.
The results showed that those who had received Darzalex were less likely to progress to active myeloma or die compared to those who didn’t receive any treatment.
So if AQUILA met its goals, why was there even a discussion?
While it’s true that the AQUILA study was properly executed, different concerns were raised at the ODAC meeting about how the results should be interpreted.
Were the patients in AQUILA truly high risk?
This depends. The definition of ‘high risk’ smoldering myeloma has evolved over time, with four different models having been developed the past 20 years. AQUILA did not use the most recent definition of high-risk in its protocol. In fact, by today’s standards, only 40% of patients in the study would be considered high-risk. Therefore, it is likely that many patients in this study were not likely to progress to active myeloma in the first place. This was in fact observed in the AQUILA study, as most patients in the control arm (who did not receive treatment) did not progress to active myeloma within two years.
What about side effects?
Several adverse reactions were associated with taking Darzalex. These included muscle pain, fatigue, diarrhea, sleep disorder, and rash. Above all, infections were twice as high in Darzalex treated patients compared to the control group. These included high grade, severe infections that required hospitalization. Given these side effects, is it worth it for patients to take Darzalex if they may not progress to MM at all?
Voices from the Front Lines: Patients Speak
For patients who testified at the ODAC, they preferred having a choice. Many discussed the stress of living with a smoldering multiple myeloma diagnosis and the empowerment they felt by being able to do something about it by getting treatment. Other patients discussed how their treatment appears to have kept their smoldering myeloma from progressing, and the relief they feel by being able to avoid active myeloma treatments and their potential toxicities.
The Final Decision – What it Means Moving Forward
After reviewing AQUILA results and hearing from doctors and patients, the ODAC voted 6 to 2 that the trial results provided enough evidence to support a favorable benefit-risk profile for Darzalex for patients with high-risk smoldering myeloma.
While this news indicates this ODAC is persuaded by current results, it does not mean the indication is approved by the FDA just yet.
The FDA will continue to analyze these study results and carefully consider the feedback from ODAC before an official decision is made.
In the meantime, more work is needed:
- We need better tools to identify truly high-risk patients without including people who will not develop active myeloma. This means we need a collaborative effort where sponsors of clinical trials contribute samples, outcomes data, and financial support to help improve existing models. The Multiple Myeloma Research Foundation® (MMRF®) has led collaborations like this in the past, and through its Multiple Myeloma Research Consortium® (MMRC®) and other initiatives, is pulling together researchers to help develop these models.
- Patients need clear, easy-to-understand information to make the right decision for their situation. For patients, education about their risk is critical. They must have the resources and tools to evaluate treatment options and make the most informed decision for their individual situation, including evaluating whether treatment or watchful waiting is the best way to go.
- No matter the treatment decision, smoldering multiple myeloma patients need support. Smoldering myeloma patients often experience distress about the course of their disease – feeling that progression to myeloma isn’t a question of if, but when. Anti-cancer treatment is not a solution for this anxiety, especially if the risks outweigh the benefits. Therefore, it is critical that mental health resources and provider communication are prioritized for smoldering patients. This will give them the support they need to move forward and continue living without the fear of their disease progressing.
For patients, if you’ve been diagnosed with smoldering myeloma, ask your doctor:
- What support is available to help me cope with a smoldering diagnosis?
- Do I meet today’s definition of high-risk?
- What are my chances of progressing without treatment?
- What side effects might I face with early treatment?
- What clinical trials are available for a patient with my level of risk?
- What’s the right path for me—treatment now or continued observation?
You can also reach out to the MMRF Patient Navigation Center for more information.
Welcome back to our coverage of the 2025 ASCO Annual Meeting. On Day 2, researchers shared more promising updates about long-term results from large, ongoing clinical trials, new treatment combinations, and patient-friendly innovations in multiple myeloma care.
CAR T-Cell Therapy: Patients Cancer-Free After 5 Years
Long-term data were presented from the CARTITUDE-1 study of the CAR-T cell therapy Carvykti® (cilta-cel) in 97 heavily pretreated patients with relapsed or refractory myeloma.
- Five years after a single infusion of Carvykti, about 1 in 3 patients were still off treatment, living cancer-free.
- Close to 36% of these cancer-free patients were initially diagnosed with high-risk disease, including chromosomal alterations and myeloma tumors in different parts of the body (extramedullary disease).
- Researchers are looking beyond genetics to figure out what has helped stall disease progression for 5 years in these patients, including their T-cell makeup.
The MMRF’s new Translational Research Umbrella (TRU) Program asks similar questions to help us understand which patients respond to specific therapies and why. Combined efforts like CARTITUDE-1 and TRU will be key in understanding the long-term benefit of Carvykti and who can benefit the most.
Above all, these are some of the most positive results we’ve seen as a treatment for heavily pre-treated.
Strength with Newer Treatment Combinations
Yesterday, we highlighted how adding Darzalex® (daratumumab) to standard three-drug treatment helps patients who aren’t getting a transplant. Today, we heard similar long-term success in another group of patients who did receive a transplant.
- In the PERSEUS trial, newly diagnosed, transplant-eligible patients received Darzalex plus Velcade®(bortezomib), Revlimid® (lenalidomide), and dexamethasone (DVRd).
- These patients were more likely to reach and stay in MRD-negative status (no detectable cancer) for up to two years compared to those on Velcade, Revlimid, and dexamethasone alone.
- 95% of patients who stayed MRD-negative had a high chance of being cancer-free at four years.
We also saw more good news for Sarclisa® (isatuximab). A study of newly diagnosed patients found that adding Sarclisa to Kyprolis® (carfilzomib), Revlimid, and dexamethasone (Isa-KRd) led to:
- Greater percentage of high-risk patients with 2 or more chromosomal alterations reaching MRD-negativity compared to those on the triplet regimen (KRd) (62% vs 20%).
- Better long-term remission after one year (52% vs. 38%)
- No increase in side effects compared to the three drug combination
Given the improvement in outcomes and no significant side effects or impact on quality of life, this study and others from yesterday confirm that four-drug combinations are the standard of care treatment across different newly diagnosed patient populations.
From Bispecifics to Trispecifics
Day 2 also offered an exciting glimpse into potential new treatments on the horizon, including JNJ-5322, a new trispecific antibody that targets two regions of myeloma cells instead of one. This new therapy could be a particularly effective treatment for patients who have just relapsed after taking a proteasome inhibitor like Velcade, an immunomodulatory drug like Revlimid, or an anti-CD38 antibody like Sarclisa.
- 100% of relapsed/refractory patients in a phase I trial responded to the drug if they had not taken any other bispecific or CAR T-cell therapies.
- Side effects were similar to those seen in other bispecific antibody treatments and included cytokine release syndrome (CRS), infections, decrease in white blood cells, and taste loss.
Now that this Phase I trial discovered an optimal dose that minimizes side effects, we look forward to watching JNJ-5322 as it moves to the next stages of testing—Phase 2 and Phase 3 trials—where more patients can access the drug.
A New Way of Giving Sarclisa Offers New Possibilities for Patient Care
Today’s last study looked at a new way to give the medicine Sarclisa using a small, wearable device called an on-body delivery injector (OBI). This device sticks to the belly and uses a tiny needle to deliver the medicine. The goal of the study was to see if this new method works just as well as the current way of giving Sarclisa, which is through an IV drip.
- Both methods worked equally well, with 71% of patients responding in both groups. Side effects were also similar between the two groups.
- The study also found that patients wearing the injector had far fewer injection related reactions and reported higher satisfaction with the treatment than patients who received an IV infusion.
- Delivery of Sarclisa took only 20 minutes using the OBI.
The wearable injector may be used in the future to improve quality of life by making treatment more comfortable, convenient, and less disruptive to daily activities. One day, it could possibly be administered in a patient’s own home instead of a treatment center.
Moving forward, it will be important to figure out what can be done to minimize cost of this new device and increase everyone’s access to it.
We’re excited by the continued momentum in myeloma research that we’ve seen at ASCO 2025. For more updates, visit themmrf.org and follow along as we track the progress that brings us closer to a cure. In this table, we’ve highlighted the key findings that could make a real difference in treatment options for people with myeloma
We’re excited to share highlights from the first day of the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, one of the year’s most important events in cancer research.
This year, researchers from around the globe shared data on breakthrough research and treatment strategies, including 150 abstracts, posters, and presentations related to multiple myeloma. On Day 1 of ASCO, we were particularly encouraged by studies that showed progress for the following key areas of unmet need:
New Possibilities for Blenrep
Blenrep (belantamab mafodotin) is a type of cancer treatment that works by combining targeted therapy with chemotherapy.
One study looked at Blenrep combined with Pomalyst® (pomalidomide) and dexamethasone (BPd) in patients who had received at least one prior treatment that included Revlimid. This study found that more patients (25%) became MRD-negative—the deepest response measurable—when treated with BPd versus Pomalyst, Velcade® (bortezomib) and dexamethasone (PVd) (5%). The study also found that patients who did not become MRD-negative still benefitted more from BPd than PVd; 32% of patients who received BPd versus 5% of patients who received PVd had a very good partial response. Another study looked at some of the common eye problems caused by Blenrep. Researchers wanted to see if giving the drug less often could help reduce these side effects, especially in frail patients. The results were promising: patients still responded well to the treatment, even with fewer doses. Very few people—less than 1%—had serious vision problems like trouble driving or reading. When Blenrep was combined with the drugs Revlimid® (lenalidomide) and dexamethasone, it appeared to be a good treatment option for newly diagnosed myeloma patients who are too weak for a transplant. These results suggest that Blenrep could be a valuable option for patients who have few treatment choices left, especially since it’s an off-the-shelf therapy that doesn’t require a hospital stay. It may receive FDA approval as early as this summer.
Better Treatment for Newly Diagnosed High-Risk Patients
Some newly diagnosed patients, especially those with high-risk chromosomal alterations, may not respond to standard therapies, but data on new drug combinations are showing hope:
- Researchers looking for a better treatment for high-risk newly diagnosed patients with the 1q21+ chromosomal alteration found that adding Sarclisa® (isatuximab) to the standard treatment (Isa-VRd) showed promising results, including higher chances of a complete response – meaning more than 95% of plasma cells are eliminated from the bone marrow.
- In a separate study that included newly diagnosed myeloma patients with multiple chromosomal alterations and stage II or III myeloma, a four-drug combination of Sarclisa, Kyprolis® (carfilzomib), Revlimid, and dexamethasone helped 87% of high-risk patients become and stay cancer free—many for 1-2 years or longer. Importantly, this study also showed that doctors could reduce the frequency of Kyprolis dosing to once a week instead of twice and still get an effective response.
Both studies indicate that adding Sarclisa to treatment protocols for high-risk newly diagnosed myeloma patients could become a new standard of treatment for this hard-to-treat patient group.
For Patients Who Can’t Get a Transplant or May Not Want One: DVRd
We saw promising updated data continuing to confirm that adding Darzalex® (daratumumab) to the standard VRd combo (Velcade, Revlimid, dexamethasone)—known as DVRd—was shown to work better than VRd alone, particularly in patients who were not eligible to have a stem cell transplant or who are delaying a stem cell transplant.
The study found that treating with DVRd vs. VRd alone led to higher MRD-negative rates (60% vs. 39% MRD-negative rates) and better outcomes overall.
These results reinforce previous findings that DVRd is an important treatment option for patients who cannot or are not currently pursuing a stem cell transplant.
New Drugs and New Drug Combinations Show Promise
In a disease as complex as multiple myeloma, identifying new treatment options is key to moving toward a cure for each and every myeloma patient. It was encouraging to hear about the results of several studies focused on uncovering new treatment options.
- In an early study of relapsed and refractory patients, 75% of patients treated with ISB 2001, a trispecific antibody targeting BCMA and CD38, responded to the therapy—including patients who have received other BMCA-targeted and/or T-cell directed therapies. The most common side effects of this new treatment included infections, cytokine release syndrome, and a reduction in white blood cells. Only one patient discontinued treatment in this study. Overall, 60% of patients treated with ISB 2001 had a very good partial response or better – meaning there was a 90% decrease in M protein levels in their blood.
- Two other studies looked at combining linvoseltamab (a new, BCMA-directed bispecific antibody similar to Tecvayli® and Elrexfio®), with Velcade or Kyprolis, to see if they could enhance how well heavily-pretreated patients responded. In both studies, at least 50% of patients had a complete response—even if they had already tried other treatments. Common side effects included CRS, infections, and a reduction in white blood cells. In future clinical trials, these two treatment regimens will be measured up against standard of care therapies for relapsed/refractory patients.
Linvoseltamab is in late stage clinical trials and is expected to receive FDA approval later this year.
We’re hopeful about the progress shared on Day 1 of ASCO 2025—and we’ll be back tomorrow with more updates for the myeloma community. Stay tuned!
In this table, we’ve highlighted the key findings that could make a real difference in treatment options for people with myeloma.
By Michael Andreini, President and CEO, Multiple Myeloma Research Foundation
Twenty-six years ago, when the Multiple Myeloma Research Foundation® (MMRF) was started, a multiple myeloma diagnosis was nearly synonymous with a death sentence. Less than 35% of people were alive five years after diagnosis, and the few treatment options that existed were largely ineffective. Today, thanks to groundbreaking research and medical advancements – many spearheaded by the MMRF and our partners – we’ve made tremendous progress in the battle against this disease. With over 15 FDA-approved therapies now available, the five-year survival rate has climbed to over 60 percent.
However, with progress comes complexity. While treatments and patient outcomes have improved, multiple myeloma remains an incurable disease with significant unmet medical needs. This year, nearly 36,000 people in the U.S. will be diagnosed. For some, existing treatments may be ineffective from the start, while others will struggle to find the right option. Furthermore, the very advancements that have transformed care are now creating new barriers to further breakthroughs, presenting new and equally pressing challenges that I’ll discuss here.
A Challenging Commercial Environment Threatens Innovation
One of the most dangerous myths about multiple myeloma is that it is now a manageable disease and no longer a pressing medical challenge because we have so many approved therapies.
Ironically, the success of new treatments has made it harder to develop additional therapies. Large biopharma companies, seeing a crowded market with limited commercial opportunity, are deprioritizing and investing less in myeloma research. Early-stage, venture-backed biotech companies, who take on the majority of new drug development, are prioritizing other disease areas like solid tumors with greater perceived clinical unmet need. Moreover, these companies face significant scientific and financial risks – challenges that are even greater in today’s difficult funding environment. That’s why the MMRF started the Myeloma Investment Fund®: to provide financial and strategic support to companies advancing novel classes of medicines and advocate for these innovative platforms to be tested in myeloma. Today, our portfolio includes 20 companies, ensuring that promising breakthroughs have the resources to reach myeloma patients.
Decreased Government Funding Puts Research at Risk
At the same time, U.S. federal funding for multiple myeloma is declining. The federal government has historically been the largest funder of biomedical research, but proposed cuts to the National Institutes of Health (NIH) put future budgets at risk.
Even before these potential cuts, funding for myeloma lagged behind. Although the National Cancer Institute (NCI) budget has grown over time, the myeloma specific budget has decreased significantly. Myeloma is almost 2% of all cancers, yet receives less than 1% of the NCI’s budget.
Funding for institutions is essential to bridge the gap to translate basic science into treatments that can be tested in clinical trials. Recognizing this critical need, the MMRF has long been the largest private funder of myeloma research, with annual investments now approaching the NCI’s myeloma-specific funding levels. Without sustained investment, innovation will stall, leaving patients with fewer options when they need them most.
Data Silos Inhibit Advances
While there have been significant advances in our understanding of myeloma, critical knowledge gaps remain. No one institution treats enough patients to represent the full patient population, and valuable clinical data is often locked away in institutional silos. This fragmentation prevents researchers from drawing broad, population-wide conclusions that could accelerate breakthroughs, including determining the most effective combinations, dosages, and treatment sequences for different patient populations.
Breaking down these data silos is essential for advancing myeloma research. Through decades of work and initiatives like the MMRF CoMMpassSM Study and Immune Atlas program, we’ve made our data freely available, aggregating patient data to identify patterns, uncover new treatment opportunities, and ultimately accelerate the path toward a cure. Our latest initiative in this space is the MMRF Virtual Lab: an online research environment that provides the most comprehensive and open-source data in multiple myeloma research on a single platform.
Patients are Being Left Behind
While the most recent novel therapies like CAR T and bispecific antibodies have transformed multiple myeloma treatment, they are not one-size-fits-all solutions. Given the genetic complexity of the disease and systemic barriers to quality care, many patients don’t benefit equally. Significant disparities persist among patients, especially those who have high-risk disease, live in rural or community settings, and are of lower socio-economic status. These disparities also impact racial/ethnic groups such as Black patients, who continue to experience lower survival rates.
While not unique to multiple myeloma, clinical trial representation remains another key barrier. Historically, trial participants have been younger, healthier, and not racially diverse. This makes it critical to conduct trials that reflect the real-world makeup of the U.S. at large to ensure new therapies work for all patients.
To help bridge these gaps, we place an emphasis on providing patient navigation services and education as we believe that patients who are informed about their disease engage actively in treatment decisions, leading to better adherence to therapeutics, quality of life, and outcomes. We also lower barriers to patient participation in clinical trials conducted by our Multiple Myeloma Research Consortium® (MMRC®) by having broad inclusion/exclusion criteria and providing financial and travel assistance to patients.
The Path Forward to a Cure
For more than 25 years, the MMRF has remained steadfast in its mission – to accelerate cures for all multiple myeloma patients. Since our founding, we’ve raised over $600 million for research, opened nearly 100 clinical trials, and helped bring more than 15 FDA-approved therapies to market, which have helped triple the life expectancy of myeloma patients and improved patient outcomes.
While the progress made is remarkable, there is still much work to be done. In times of uncertainty, organizations like the MMRF – unbound by federal funding constraints and able to serve as an unbiased third party – play a unique and critical role in advancing research that might not otherwise be possible. The MMRF will continue to lead with urgency, breaking down barriers and working with the academic, industry, and patient communities to drive meaningful progress and impact in myeloma.
Welcome! We’re here to recap the latest news in Minimal Residual Disease (MRD) status from the Myeloma MRD 2025 Meeting that kicked off Friday, April 11, at the Sylvester Comprehensive Cancer Center in Miami. The MMRF has been part of this meeting since its inception, participating in a number of panels, presentations, and thoughtful discussions along with clinicians, researchers, companies, advocates, patients, and caregivers on the state of MRD testing.
MRD is an important part of monitoring myeloma after treatment. It measures how many cancer cells are still in the bone marrow. If someone is MRD-negative, it means the number of cancer cells is so small that that they are nearly undetectable.
Here are the major takeaway points from the MRD Meeting:
- While sustained MRD negativity is closely tied to favorable outcomes, MRD doesn’t have the same predictive value for every patient. In fact, many patients have a strong and durable response to treatment without ever becoming MRD negative.
- Clinicians and biopharma companies are now using MRD status as an important way to measure how well treatments are working in clinical trials. This is based off the 2024 FDA Oncology Drug Advisory Committee’s positive vote about using MRD in clinical trials as an intermediate endpoint to show that treatment is working.
- Research is being done to better understand the role of MRD in making treatment decisions and to develop more convenient ways to measure MRD status (for example using a blood sample versus a bone marrow sample)
Let’s take a closer look!
Knowing your MRD status is helpful, but there is much more to the story
Reaching MRD negativity can be good news for patients, and many clinical trials showed that sustained MRD negativity is closely tied with favorable outcomes.
However, there are several important points we have to remember. For one, these studies and others showed that MRD negativity could be reached no matter what treatment patients were on. For example, studies that compared triplet versus quadruplet myeloma therapies both showed a significant amount of patients reaching MRD negativity.
Secondly, patients may never achieve MRD negativity but still have favorable outcomes. For example, people with monoclonal gammopathy of undetermined significance (MGUS) have a higher number of plasma cells and are not MRD negative. Yet, they rarely progress to myeloma. Some myeloma patients who are very good partial responders or complete responders and have clearly detectable MRD can also stay in remission for a long time.
Lastly, being MRD negative shouldn’t be confused with being cured. In several studies, we saw that patients who were MRD negative could still relapse to being MRD positive over time.
Taken together, these examples show that MRD status is only one piece of a larger puzzle.
To fully understand a patient’s prognosis and make the best treatment decisions, their care team looks at more than just MRD status. They also consider genetic changes in their myeloma, how many treatments they’ve had before, and how well their body handles those treatments. Also, more research is still needed to figure out how well patients will do if they do not reach MRD negativity, and why some MRD negative patients relapse.
For all these reasons, the use of MRD data to inform decisions for individual patients is still an area of active research.
The Growing Importance of MRD in Myeloma Clinical Trials
One discussion in Miami centered around the FDA Oncologic Drugs Advisory Committee (ODAC) agreement that MRD status could be used as an intermediate endpoint in clinical trials. But what does this actually mean for patients?
For new myeloma treatments to be approved, clinical trials must first reach what are called primary endpoints. These are outcomes that can tell if the new treatment was effective or not. Many myeloma therapies rely on primary endpoints like progression free survival (PFS), or overall survival (OS). While these endpoints tell us a lot , they can take a long time to determine if a treatment is effective and safe.
Fortunately the FDA has been open to reducing that time by using an endpoint that could predict PFS. Now, MRD data has come into play because the latest clinical evidence shows that MRD can be a useful tool for predicting if a clinical trial will show a PFS benefit. And the MRD data for a clinical trial can be available years before the PFS data.
Tying this back to the ODAC in 2024, this group recommended that MRD data be considered an intermediate endpoint that the FDA could use to allow a biopharmaceutical company to market a new treatment while PFS and OS continue to be collected. This could help bring new myeloma treatment options to patients because clinical trials reach the MRD endpoint more quickly.
Given the emerging use of MRD data in clinical trials, it is even more important to understand safety and PFS in order to know what is best for patients.
Making MRD Testing More Readily Available
The good news about MRD testing? It can be reimbursed, it is part of the National Comprehensive Cancer Network (NCCN) guidelines for measuring a response to treatment, and it is available at academic medical centers across the country.
The bad news? It is not widely available in the community, for a variety of reasons.
Finding out MRD status takes time. Not only does a patient need to have a bone marrow biopsy, but that sample must be taken to a lab where very sensitive tests are performed to figure out how many myeloma cells they have. This entire procedure may not be available to everyone –financial, transportation, and other logistical barriers are in the way for patients, and community providers may not have the resources needed to perform MRD testing.
Could this all change?
During the end of the workshop, presenters discussed new, innovative ways to make MRD testing easier and more accessible. Many of the techniques discussed looked to bypass the need for a bone marrow biopsy, and instead, figure out the number of myeloma cells using DNA or protein collected strictly from blood samples.
Why is this important?
Improved availability and lowering logistical barriers for patients and providers could pave the way for frequent MRD testing. This could improve the prognostic information for patients and doctors, and spur on more research to optimize the use of MRD in trials and for patients.

How did you get involved with MMRF?
After being diagnosed with multiple myeloma and not showing improvement from at least four different courses of treatments, I was given a medication to treat my disease because of my expert medical team’s knowledge of a drug that was originally used for leukemia. If it wasn’t for research, their expertise and ongoing discussions with colleagues, this medicine would never have been recognized for treating myeloma. It worked! I was showing data that my aggressive form of myeloma was finally responding to a treatment. Before being diagnosed, I wasn’t even aware of the fact that people didn’t always respond to certain treatments. This opened a new light in my thinking. Imagine if there was no research! We need new forms of treatment, and the only way to get this is through funds to employ scientists and expand research to find new methods of treatment. MMRF helps provide this kind of research so that we can reach more people who have myeloma and give them a fighting chance.
Why did you choose to participate in the MMRF Walk/Run?
I want people to look at me and be inspired by the fact that if they see me participating in the MMRF Walk/Run they can persevere as well. Perseverance is needed to meet goals so they are more than just dreams. If I am visible to others, there can be hope for so many people to live a life with quality after a myeloma diagnosis. My Multiple Myeloma specialists at Tampa General Hospital, Dr. Borrello and Jen Hanle, have been an incredible source of support during some of the most challenging times in my life. Their dedication and involvement with the MMRF Walk/Run is a big part of why I choose to participate. I want to give back to them in any way that I can and thank them by living a life with meaning. Supporting their efforts to raise awareness and funds for research through MMRF’s Walk/Run is one way that I can say thank you and support them. Some people may know me or see photos of me during my myeloma journey. They could see me in photos during my difficult time with challenges such as using a walker, wearing a patch on my eye, wearing an orthopedic collar on my neck, and seeing me now able to Walk/Run in this event. To see me smiling and grateful each day I awake and actively participate in the world around me can be an inspiration and motivation to keep going. I can reach more people during this event than I ever thought possible.
The Spirit of Hope is given to individuals/groups who inspire hope and show extraordinary commitment to the MMRF. What does being given the award mean to you?
Being given the honor of The Spirit of Hope is a way of telling me I am doing a good job inspiring others along this journey to persevere with hope. It will help me to persevere and to continue to motivate others who have been affected by myeloma.
Please share any stories that have given you strength.
When I heard my diagnosis for the first time, believe it or not, I felt a sense of comfort. Perhaps it was because my dad was diagnosed with myeloma several years ago, and I knew that he led a good life for quite a while despite it. I also knew a little bit about the disease, and that felt a little less scary than the menu of other options that I didn’t know as much about. I tried hard to focus on the fact that in the time my dad was diagnosed until the time I was diagnosed, so many new treatments were discovered. If this was the case, then I had to focus on how many new treatments were going to be found in my lifetime. Maybe there would even be a cure.
I also felt deep down that this was not my time for my family and me to mourn but rather to rally and persevere. Yet, my family (although the best supporters of all time) were secretly mourning me. I saw it in their eyes. This was the most difficult obstacle for me to see, even more than the treatments that weren’t working or the pain that I endured. How could I change that? I needed to change their response from supporter to believer. They needed to believe that I could pull through this with the help of a superb medical team, research, resistance to the temptation to give up, and a little luck.
During my professional life my role in the New York City School System was to give strategies to other teachers and principals to put forth their best work and make changes for the better. I was a coach. I motivated others and gave them methods and strategies to help them achieve their goals. I realized I could do this with my illness. Now was my time to teach others that if I could have the strength to persevere until I reached success, perhaps they could too. This wasn’t always easy because my data did not always show improvement. There was evidence that I was not in a good place for quality and survival at different points after my diagnosis. I refused to show the world that I was merely a statistic. I remembered a strategy I knew that could be used in persevering for success. Dress like you already are a success, and play the role you want to be. I was determined not to look like my data. I would wake up, and most mornings, I’d start to feel more comfortable when I saw my image in the mirror. I was dressing like I already reached my goal — remission. Mornings were my time. The sun peeping in was and is a sign of hope to me. It is my reminder to get up and do something positive for myself and for those I love. Walk. Stretch. Be creative. Cook a new recipe. Read. Dress in your best clothing. Do something that helps someone else. I did it all. I’d walk to my closet and find my best outfit to wear. I dressed like I was enjoying a quality life.
When I was told I had to isolate, I kept thinking how fortunate I am for finally qualifying for my stem cell transplant and for living in a time with technology such as FaceTime and Zoom so I can continue to see the people I love. As I showed the world my response to myeloma, they began to respond similarly. They believed I could persevere. It fed on itself. At the same time I was doing this, my treatment plan was changed many times. Finally, there was one that was beginning to show improvement towards a positive outcome, which in turn gave me even more of a positive outlook.
Some people believe in blind faith. I believed I needed to sift out negative thoughts and show the world my positivity. Demystify cancer! Demystify chemo! I began going outside with a bald head dressed to the nines. I remember being told that some people are beautiful with or without hair and I was determined to show the world this is true. I felt a different kind of beauty. This was a beauty that people needed to see. It comes from your inside and radiates into the world like sunshine. I kept saying to myself, “I want to show people that we must celebrate life every single day and find things to be grateful for. I have many things to be grateful for — a husband, daughters, grandsons, true friends, hobbies, and a career I love. I must show the world who I am. I am not just a statistic. I am not a box of cookies with an expiration date. I am a person living a purposeful life.
I may not be able to change my diagnosis, but I can change my response to it. Maybe that will help others to change their response as well.


How did you get involved with the MMRF?
I was diagnosed in July 2018 with multiple myeloma. After a few office visits, I learned about the organization offering support and research information concerning this dreaded illness.
Why did you choose to participate in the MMRF Walk/Run?
It was incumbent upon me to do all that I could do within my power to help support others dealing with myeloma. The Walk/Run felt personal and was a small but important contribution from me.
The Spirit of Hope is given to “individuals/groups who inspire hope and show extraordinary commitment to the MMRF.” What does being given the award mean to you?
To say that I am honored is truly an understatement. When I was approached about this, I became extremely emotional that the team would think of me or see my journey and felt it was worthy of sharing. This means the world to me. While I was going through my chemo treatment, I would try to talk to and encourage people that I met in the waiting area or in the lab. Being given this award means I am accountable. I am accountable because I have been given the opportunity to live, to enjoy my family, to enjoy life, to work, to play, and to vacation. I feel that it is my duty to make sure that the medicines, services, tools, and information that were used for my benefit are shared by me with others.

How have you found perseverance in light of obstacles? Please share any stories that have given you strength.
I had no idea what this trial and treatment were going to look like. I was extremely nervous. But being a woman of faith, I trust God implicitly! He was a major part of my healing process. Reading my Bible, taking walks, and having a dedicated support system are all part of the journey. It is truly important to have strong people in your corner who will help pull you up when you don’t feel like getting up. Once up, you can take one step, and then two, to propel you forward. Getting out and feeling the gentle breeze and the sun on your face can be a game changer because it makes the journey seem a little lighter; it does not go away. It just becomes manageable.

Do you have a favorite mantra, quote, or lyric that gives you strength?
Yes! My favorite mantra throughout my journey and still today is, “ALL IS WELL!” maintaining a cheerful outlook is key. Keeping your mind healthy helps to keep your body healthy as well.
Anything to add?
I would like to give a heartfelt thank you to my entire Atrium clinical staff, starting with Doctor Atrash, Claire, Sydney, the lab, and a host of nurses and MA’s on the fourth floor at Levine Cancer Center that helped get me through my journey. I am here and able to celebrate this moment because of all of you.

Several presentations at the 2024 American Society of Hematology (ASH) Annual Meeting relayed new and updated study data in multiple myeloma (MM), along with significant advancements in the treatment of
- Smoldering multiple myeloma (SMM)
- Prognostic indicators
- Newly diagnosed MM (NDMM)
- Maintenance therapy
- Minimal residual disease (MRD) testing
- Relapsed/refractory MM (RRMM)
- AL amyloidosis
Advances in SMM Management
Ongoing research of SMM treatment is focused on early intervention and observation with monitoring, identification of patients at high risk of progression, and novel treatment approaches. Two studies addressed these issues.
Phase 2 EMN15/HOVON147 Study: KRd vs Rd
- Intervention: Lenalidomide and dexamethasone (Rd) ± carfilzomib (K) as induction therapy
- Patient population: 58 patients with high-risk SMM (HR-SMM) identified by MAYO and/or PETHEMA* criteria
- Efficacy: At a median follow-up of 34 months, KRd significantly improved
- MRD negativity, next-generation flow cytometry [NGF]; 10-5 (57% vs 5%; odds ratio [OR]=0.04, 95% confidence interval [CI], 0.00–0.28; P<0.001)
- Progression-free survival (PFS) (hazard ratio [HR]=0.08, 95% CI, 0.02–0.35; P<0.001)
3-year PFS of 94% vs 40%. MRD negativity after 4 cycles was not significantly better with KRd (37% vs 14%; OR=0.27, 95% CI 0.06–1.12; P=0.07).
- Safety: Although KRd was associated with higher rates of grade 3/4 adverse events (AEs) (74% vs 53%), the combination resulted in fewer treatment discontinuations due to progression (6% vs 36%)
- Takeaway: The authors concluded that the addition of K as induction is “feasible and safe in patients with HR-SMM.” It is important to note that treatment of HR-SMM is still an evolving area where high-risk patients are not predictably identified; the benefit of treatment should outweigh its risks
*MAYO criteria: bone marrow plasma cells ≥10%, serum M protein ≥3 g/dL, and serum free light-chain ratio <0.125 or >8; PETHEMA criteria: presence of ≥95% abnormal plasma cells (presence or absence of CD38, CD56, CD19, and/or CD45), and immunoparesis, defined as a reduction (below the lower normal limit) in the levels of 1 or 2 of the uninvolved immunoglobulins.
Phase 3 AQUILA Study of Daratumumab
- Intervention: Daratumumab vs monitoring
- Patient population: HR-SMM (defined as clonal bone marrow plasma cells [BMPCs] ≥10% and ≥1 risk factor [serum M protein ≥30 g/L, IgA SMM, immunoparesis with reduction of 2 uninvolved Ig isotypes, serum involved:uninvolved free light chain ratio ≥8 and <100, and/or clonal BMPCs >50% to <60%])
- Efficacy: At a median follow-up of 65.2 months,
- PFS was significantly improved with daratumumab (HR=0.49; 95% CI, 0.36–0.67; P<0.0001), with a median PFS not reached in the daratumumab group vs 41.5 months in the active monitoring group
- The overall response rate (ORR) was significantly higher with daratumumab (63.4% vs 2.0%; P<0.0001), with a prolonged median time to first-line MM treatment (not reached vs 50.2 months; HR=0.46; 95% CI, 0.33–0.62; P<0.0001)
- Safety: Grade 3/4 AEs were more common with daratumumab (40.4% vs 30.1%), but the treatment was generally well tolerated, with low discontinuation rates (5.7%). The most common AE was hypertension (5.7% vs 4.6%).
- Takeaway: The authors noted that these findings “strongly support the benefit of early intervention with daratumumab monotherapy vs active monitoring.”
Functional Risk and the Role of 24-Hour Urine Tests
Two studies highlighted important aspects of prognostic indicators: one addressed functional high-risk disease status and the other assessed the value of routine 24-hour urine assessments.
Functional High Risk
Functional high-risk status could be a useful tool for identifying patients with poor prognosis, according to one analysis. Functional high-risk MM, characterized by disease progression or death within a year of initial treatment, is a poor prognostic subset regardless of high-risk cytogenetic features at diagnosis. Of the 228 functional high-risk patients identified from the Multiple Myeloma Research Foundation (MMRF) CoMMpass study, 165 were categorized as high risk and 63 as standard-risk (ie, patients with or without high-risk features [t(4;14), t(14;16), t(14;20), 1q amplification (amp1q), 17p deletion (del17p), TP53 mutations, or ISS-stage III disease]). The median overall survival (OS) was 13.2 months for the standard-risk group and 11.6 months for the high-risk group (P=0.29). Primary refractory disease was prevalent in both groups, with OS significantly shorter for patients with high-risk vs standard-risk disease (5.0 vs 7.8 months; P=0.048).
Despite differences in second-line treatment patterns (standard-risk patients received more triplet regimens; high-risk patients relied on doublets), response rates were similar, with median OS for relapsed patients 27.1 months vs 20.2 months (P=0.22 standard- vs high-risk). Overall, functional high-risk patients had an OS <24 months regardless of the presence of high-risk features at diagnosis.
This study confirms that many high-risk disease features do not present with other known high-risk features at diagnosis. More research into reliably identifying patients with high-risk features is needed.
Urine Testing
A secondary analysis of the BMT CTN 0702 (STaMINA) trial evaluating whether 24-hour urine testing adds value to MM response assessments based on International Myeloma Working Group (IMWG) criteria found that removing 24-hour urine testing requirements from response criteria resulted in changes to <1% of patient responses, with no impact on PFS prediction at any response depth. Median PFS for traditional and urine-free criteria was identical across response categories, including 63.0 months for complete response (CR) and 49.6 months for very good partial response (VGPR). Urine-free IMWG criteria remained highly prognostic for PFS (P=0.006; HR=1.05–1.36).
The authors concluded that urine-free criteria “reduce time, toxicity, and discomfort for patients, particularly those with limited dexterity,” though they also noted that these assessments remain critical in specific cases, such as AL amyloidosis or urine-only measurable disease.
Advances in NDMM Induction Therapy
Several studies highlighted the evolution of induction therapy for improving survival and quality of life for patients with NDMM. Current standard-of-care (SOC) regimens, such as quadruplet therapy incorporating daratumumab or isatuximab with bortezomib-lenalidomide-dexamethasone (VRd), continue to demonstrate superior MRD negativity and PFS regardless of autologous stem cell transplant (ASCT) eligibility, making these regimens an appropriate option for a majority of patients. Data on additional investigational therapies, including teclistamab- and belantamab-based regimens, were also presented.
Phase 3 GMMG-HD7 trial: Isatuximab-VRd
- Intervention: VRd ± isatuximab as induction therapy
- Patient population: Transplant-eligible (TE) NDMM patients
- Efficacy: After a median follow-up of 47 months, compared with VRd alone, isa-VRd:
- Significantly improved PFS (HR=0.70, 95% CI, 0.52–0.94; P=0.0184), with 3-year PFS rates of 83% vs 75%, respectively
- Provided consistent PFS benefits across most baseline characteristics through subgroup analyses, though patients with high-risk cytogenetics and poor performance status showed no significant improvement. Multivariable analysis indicated a benefit for isa-VRd (HR=0.64, 95% CI, 0.47–0.86, P=0.004)
- Takeaway: The MRD negativity benefit with isa-VRd is consistent with previous reports
Phase 3 IMROZ trial: Isatuximab-VRd
- Intervention: VRd ± isatuximab
- Patient population: Transplant-ineligible (TI) NDMM patients
- Efficacy: Compared to VRd alone, isa-VRd
- Achieved higher rates of MRD-negative CR (56% vs 41%; P=0.003; next-generation sequencing [NGS], 10-5) and sustained MRD negativity for ≥12 months (47% vs 24%)
- Had shorter median time to MRD negativity (14.7 months vs 32.8 months)
- Demonstrated greater depth of MRD negativity and more frequent positive-to-negative MRD conversions during maintenance over 36 months, correlating with PFS benefits
- Takeaway: These findings support the benefit of adding isatuximab to VRd as initiation therapy, as well as during maintenance in TI NDMM patients
Investigational Quadruplets
Notable findings regarding investigational quadruplets in NDMM were reported from two studies.
The phase 2 MajesTEC-5 study evaluated the use of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in combination with daratumumab-lenalidomide-dexamethasone (DRd) or D-VRd as induction therapy for patients with TE NDMM. Out of 49 patients, all assessable patients (n=35) achieved MRD negativity (NGF, 10⁻⁵) after completing 3 treatment cycles, with sustained MRD negativity in those completing 6 cycles. Despite a high rate of cytokine release syndrome (CRS) events (65.3%, all grade 1/2), no cases of neurotoxicity or treatment discontinuation due to AEs were reported. The regimens demonstrated robust clinical efficacy with a manageable safety profile, suggesting their potential for improving long-term outcomes in this setting.
Promising results were reported from the phase 1 DREAMM-9 trial of belantamab mafodotin (belamaf) combined with VRd in TI NDMM patients. Across 8 dosing cohorts involving 108 patients, ORR was high, ranging from 71% to 100%, with the CR rate reaching 92% in certain cohorts. MRD negativity (NGS, 10-5) was most notable in higher-dose groups, achieving rates up to 75% in patients with CRs. Ocular events were the most common AE, with grade 3+ keratopathy and visual acuity scale AEs affecting up to 92% of patients in higher-dose groups, though longer dosing intervals reduced the frequency and delayed the onset of these events.
Maintenance Therapy
Also reported at ASH 2024 were findings from two studies on the use of maintenance therapy in MM, including duration of maintenance therapy, role of combination therapies vs single-agent maintenance, MRD status for informing therapy decisions, and the role of novel agents.
Phase 3 AURIGA Trial: Daratumumab + Lenalidomide vs Lenalidomide Alone
- Intervention: Lenalidomide ± daratumumab as maintenance therapy
- Patient population: TE NDMM patients who were MRD-positive after ASCT
- Efficacy: The addition of daratumumab significantly increased the MRD-negative conversion rate (10-5) at 12 months across subgroups, including patients <65 years (49.2% vs 19.7%; OR=3.95, 95% CI, 1.76–8.85) and ≥65 years (52.6% vs 17.5%; OR=5.24, 95% CI, 1.86–14.74), as well as in high-risk cytogenetic subgroups (eg, 43.8% vs 13.3%; OR=5.06, 95% CI, 1.43–17.88). PFS also favored D-R
- Safety: Grade 3/4 treatment-emergent AEs were more frequent with D-R than R, especially in Black patients (75.0% vs 66.7%) and patients <65 years (76.3% vs 63.8%)
- Takeaway: Adding daratumumab to lenalidomide maintenance improves outcomes for NDMM MRD+ patients following ASCT
Teclistamab + Lenalidomide vs Teclistamab vs Lenalidomide
- Intervention: A phase 3 safety run-in study of teclistamab combined with lenalidomide vs teclistamab vs lenalidomide maintenance therapy alone
- Patient population: NDMM patients post ASCT
- Efficacy: 100% MRD-negative CR rate at 12 months in Cohort 1, with all MRD-positive patients achieving MRD-negative status during treatment. Teclistamab was stopped after 13 cycles of treatment if ≥CR was achieved
- Safety: Grade 3/4 neutropenia and infections were common. Neutropenia occurred less frequently with reduced teclistamab dosing schedules; infections followed a similar trend: CRS occurred in 43.6% of patients but was mild (6.4% grade 2; no severe cases), and no immune effector cell-associated neurotoxicity syndrome (ICANS) was reported.
- Takeaway: Teclistamab alone or in combination with lenalidmoide can be safely administered as maintenance therapy following ASCT in NDMM
MRD Testing
MRD and Treatment Cessation
A prospective study evaluated whether discontinuing lenalidomide maintenance therapy after 3 years of sustained MRD negativity (NGF) might be feasible and safe in NDMM patients following ASCT. MRD status was assessed in patients who had achieved stringent CR and then at 6, 12, 24, and 36 months after the initiation of lenalidomide maintenance. Patients who had at ≥3 consecutive MRD-negative results and had received at least 36 months of maintenance discontinued lenalidomide maintenance only if they had also achieved imaging MRD negativity (via PET/CT scan). MRD was performed every 6 months thereafter. At 3 years, 26.3% of patients (N=194) achieved sustained MRD negativity in bone marrow and imaging and discontinued maintenance. Over a median follow-up of 32 months after discontinuation, 96% of these patients remained MRD negative at 6 months, with a gradual decline to 86% at 3 years. Median PFS for this cohort was 74 months (95% CI, 38–104 months).
According to the authors, “sustained MRD negativity after ASCT and completion of 3 years of lenalidomide maintenance may guide the safe discontinuation of maintenance” They also noted, however, that further validation in randomized trials is needed.
MRD After Induction
Several studies demonstrated that sustained MRD negativity with maintenance therapy is associated with improved outcomes. Whether TE or TI, patients showing deeper responses to therapy had prolonged PFS and OS, regardless of therapeutic approach.
- Study: Phase 3 CEPHEUS trial
- Intervention: VRd ± daratumumab in NDMM patients ineligible for or deferring transplant
- Efficacy: At a median follow-up of 58.7 months, compared to VRd alone, dara-VRD
- Improved MRD negativity rates (NGS, 10-5), 60.9% vs 39.4% (OR=2.37; 95% CI, 1.58–3.55; P<0.0001)
- Improved sustained MRD negativity at ≥12 months, 48.7% vs 26.3% (OR, 2.63; 95% CI, 1.73–4.00; P<0.0001)
- Was associated with over 80% of patients remaining progression-free at 54 months
- Takeaway: D-VRd is a potential new SOC for TI NDMM patients
Results from another study suggested that, in NDMM patients treated with quadruplet therapy and ASCT, MRD progression (defined as a ≥1-log10 increase in MRD burden) was associated with a median time of 10.1 months to IMWG-defined progression.
- 78% of patients (N=216) achieved MRD negativity at the 10⁻⁵ threshold (NGS), with 56% ceasing therapy upon confirmed negativity
- MRD progression was identified as an indicator of imminent progression, yielding a 1-year survival free of second-line failure rate of 55.6%, compared to 35% for progression not preceded by MRD progression
- Notably, MRD progression outcomes, including a 2-year OS rate of 78%, were comparable to IMWG-defined progression
The authors concluded that MRD progression challenges reliance on paraprotein-based progression criteria, suggesting the need for earlier intervention and therapies with novel mechanisms of action.
- Study: Phase 3 GMMG-HD7 trial
- Intervention: VRd ± isatuximab
- Patient population: TE NDMM patients
- Efficacy: Compared with VRd alone, isa-VRd resulted in
- Significantly higher MRD negativity rates post induction (55% vs 41%), and continued MRD negativity further improved PFS (HR=0.41, 95% CI, 0.25–0.65; P<0.001)
- Significantly longer PFS in MRD-positive patients (HR=0.64, 95% CI, 0.43–0.96; P=0.03)
- Takeaway: Adding isatuximab to standard VRd therapy achieves deeper responses and improves outcomes in NDMM
An analysis of the GEM2017FIT trial demonstrated that peripheral residual disease (PRD) assessed by mass spectrometry and MRD assessed by NGF have significant prognostic value for PFS in older TI NDMM patients.
- Both PRD and MRD negativity were strongly associated with improved PFS (PRD: HR=29, P<0.0001; MRD: HR=0.31, P<0.0001), with concordant results in 79.6% of cases
- Patients with negative PRD or MRD had superior outcomes across different fitness (Geriatric Assessment in Hematology scale ≤20 or >20) and cytogenetic risk categories, with HR PRD+/MRD+ patients achieving worse outcomes (HR=12, P<0.0001)
According to the researchers, these findings support the integration of PRD evaluation into routine clinical practice to complement MRD, improving risk stratification and informing treatment strategies for older MM patients. Taken together, these findings highlight the role of PRD and MRD negativity as a robust prognostic marker and treatment goal in NDMM.
Biomarkers and Sequencing Strategies for Relapsed MM
Several studies suggested that pretreatment biomarkers and treatment sequencing are important considerations for optimizing therapeutic responses and minimizing toxicity in relapsed MM.
Prior ASCT for Chimeric Antigen Receptor (CAR) T
MM patients had significantly shorter PFS with BCMA-directed CAR T-cell therapy if they had previously received an ASCT, according to one report. A study involving 104 patients indicated that although use of HDM/ASCT did not influence the CR rate (P=0.42), patients treated with prior HDM/ASCT experienced a shorter median PFS with CAR T therapy (9.5 vs 21 months; P=0.01). Multivariate analysis confirmed this association (HR=2.17, 95% CI, 1.25–3.74; P=0.006), independent of other factors such as high-risk cytogenetics or prior treatments. Notably, the timing between HDM/ASCT and CAR T did not influence PFS, and prior HDM/ASCT had no effect on OS, CRS, or ICANS. The authors conclude that these findings may help inform treatment sequencing in MM to optimize outcomes.
Toxicity and Durable Response to CAR T-Cell Therapy
Factors influencing toxicity and durable responses were identified in a comprehensive analysis of pretreatment biomarkers in 108 patients receiving idecabtagene vicleucel (ide-cel) for RRMM. High inflammatory markers (eg, ferritin, IL-6, IL-15; P<0.05) at baseline and elevated plasma cell burden (≥50%; P=0.02) were associated with a higher risk of ICANS, whereas higher cell doses correlated with increased CRS severity (median dose 440 vs 411 × 10⁶; P=0.01). Durable responses (defined as PFS at ≥9 months) were linked to favorable bone marrow profiles, including higher CD4:CD8 ratios and increased cytotoxic natural killer and central memory CD8+ T cells. Conversely, nondurable responses were associated with elevated inflammatory markers, prior BCMA exposure, and high levels of myeloid-derived suppressor cells (P=0.0025). Given these findings, pretreatment evaluation may help optimize patient selection and improve outcomes with ide-cel therapy.
Prior BCMA Exposure for Bispecifics
Findings suggest that response to teclistamab may depend on the timing and type of prior BCMA-directed therapy, offering insights into treatment sequencing in RRMM. In a multicenter study evaluating teclistamab in RRMM, prior exposure to BCMA-directed therapy was associated with a lower ORR (51.4% vs 61.5%; P=0.012) and shorter PFS (median 4.6 vs 8.2 months; P=0.017) than was seen in patients not previously treated with BCMA-directed therapies. Although prior BCMA-directed therapy was not independently predictive of PFS (HR=1.25, 95% CI, 0.95–1.64; P=0.1), waiting >8.7 months between BCMA therapies was linked to superior PFS (8.1 vs 2.5 months; P=0.001). Toxicity profiles were comparable between groups, but grade 3 thrombocytopenia occurred more frequently in the BCMA-exposed cohort (10.7% vs 6.6%; P=0.08).
Early RRMM: Innovations and Emerging Standards
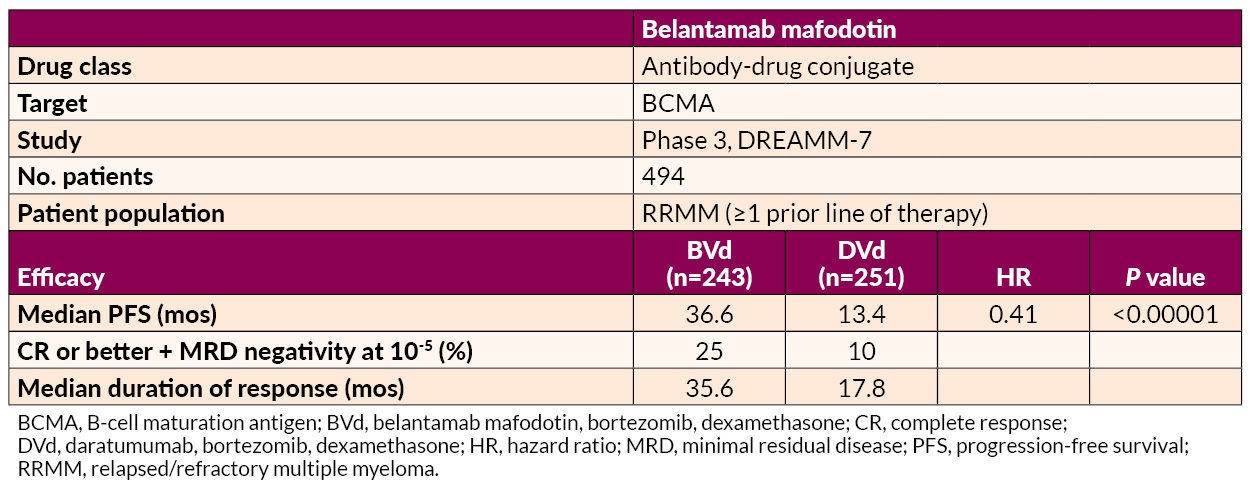
Several studies on the treatment of early RRMM were presented. Belantamab mafodotin–based regimens demonstrated superior PFS to and deeper responses than daratumumab-based combinations, offering a potential new option for first-relapse treatment.
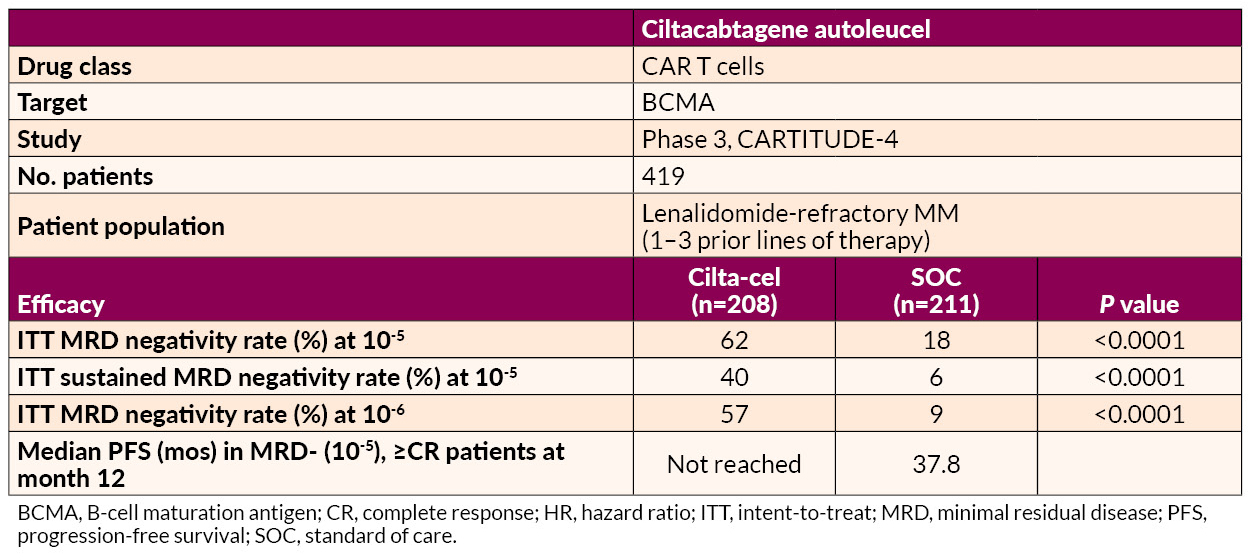
Ciltacabtagene autoleucel (cilta-cel) delivered significant benefits in MRD negativity and prolonged PFS relative to SOC in lenalidomide-refractory patients.
Cereblon E3 Ligase Modulator (CELMoD) and Elranatamab
Mezigdomide in combination with bortezomib or carfilzomib achieved high response rates (up to 85.7%) and durable responses (median PFS up to 17.5 months) across dose-expansion cohorts. Mezigdomide in other novel combinations, such as with agents targeting key oncogenic pathways (eg, MEK and BET inhibitors), displayed promising efficacy with manageable toxicity profiles.
Elranatamab combined with carfilzomib and dexamethasone demonstrated promising efficacy with a 100% ORR and manageable safety signals, including no dose-limiting toxicities in early-phase trials.
Innovative Approaches in Late RRMM Treatment
Several novel approaches to late treatment of RRMM with CAR T cells, including BCMA-targeted anitocabtagene autoleucel (anito-cel) and HBI0101 and bispecific antibodies, also showed promise.
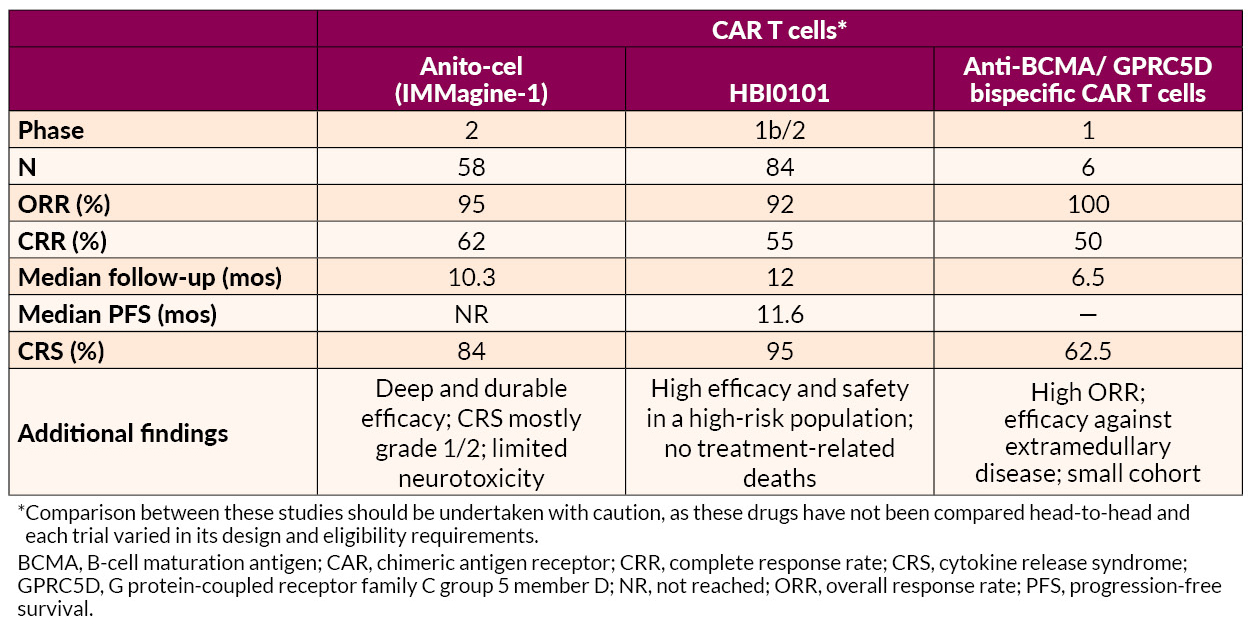
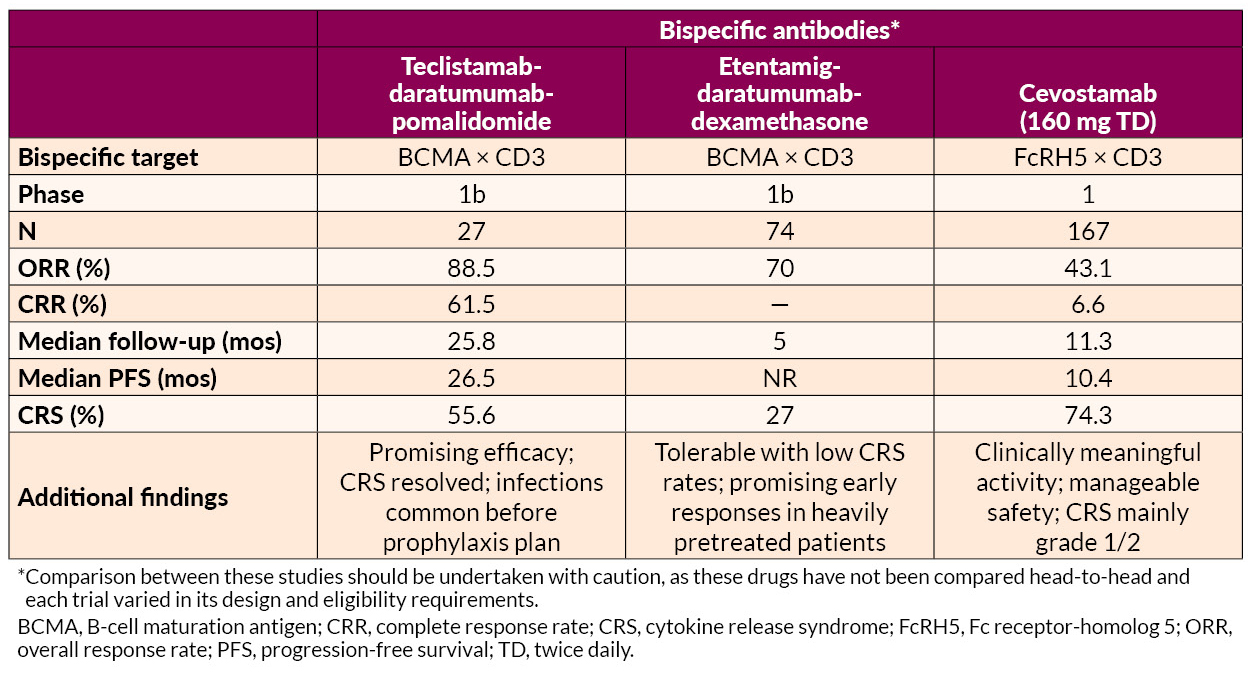
AL Amyloidosis Advances
AL amyloidosis is a plasma cell disorder and occurs in 10% to 15% of MM patients. Studies presented on AL amyloidosis showed benefits for novel drug combinations and CAR T-cell therapy. In addition, one analysis suggested that further development of therapies targeting deposited amyloid fibrils may be useful for addressing cardiac dysfunction associated with AL amyloidosis.
Phase 3 ANDROMEDA trial
- Intervention: Bortezomib + cyclophosphamide + dexamethasone (VCd) ± daratumumab
- Patient population: Newly diagnosed AL amyloidosis patients
- Efficacy: Adding daratumumab to VCd resulted in
- Higher hematologic CR rates (59.5% vs 19.2%; OR=6.03, 95% CI, 3.80–9.58; P<0.0001)
- Prolonged major organ deterioration PFS (MOD-PFS; HR=0.44, 95% CI, 0.31–0.63; P<0.0001)
- Prolonged OS (HR=0.62, 95% CI, 0.42–0.90; P=0.0121)
The median MOD-PFS was not reached for daratumumab-VCd vs 30.2 months for VCd. The 5-year survival rates were 76.1% with daratumumab-VCd vs 64.7% with VCd. Cardiac and renal responses were approximately doubled in the daratumumab-VCd group.
- Safety: Grade 3/4 AEs included lymphopenia and pneumonia
- Takeaway: According to the researchers, the findings reaffirm “this regimen’s position as the only SOC in this difficult-to-treat disease”
Phase 2 ISAMYP Study
- Intervention: Isatuximab + pomalidomide + dexamethasone (IsaPd)
- Patient population: 41 patients with relapsed or suboptimal-response AL amyloidosis
- Efficacy:
- 60% achieving ≥VGPR after 1 cycle
- 80% reaching ≥VGPR after 6 cycles
- 51% achieving CR
Treatment responses were observed early on, with a median time to initial hematologic response of 1 week and ≥VGPR within 4 weeks, including in patients with poor initial responses.
- Safety: Grade 3/4 AEs were observed in 80.5% of patients; most were manageable with dose adjustments or supportive care
- Takeaway: Rapid, deep hematologic responses were achieved with IsaPd
Phase 2 Multicenter Study for t(11;14) AL Amyloidosis
- Intervention: Venetoclax + dexamethasone (ven-D)
- Patient population: Newly diagnosed t(11;14) AL amyloidosis patients
- Efficacy:
- The CR + VGPR rate at 3 months was 58.6%, including CR in 27.6%
- The best hematologic response at any time, a composite of the CR + VGPR, was 62.9%, and the CR rate was 37.1%
- Organ responses at 6 months included improvements in cardiac function (35.0%) and renal function (90.9%)
- Safety: 3 cardiac-related deaths occurred and 6 patients discontinued due to lack of efficacy. Severe AEs such as lymphopenia and liver dysfunction affected 5.6% of patients
- Takeaway: ven-D offers rapid and high-quality hematologic responses with manageable safety risks
Another study found that the novel anti-BCMA-targeted CAR T-cell therapy HBI0101 demonstrated high efficacy and acceptable safety in 16 heavily pretreated patients with relapsed/refractory AL amyloidosis.
- Hematologic responses were present in 94%, with 75% attaining CR
- MRD negativity (flow cytometry; 10-5) was observed in 64% of evaluable patients
- Organ responses occurred in 50% of evaluable cases, with improvements noted in cardiac and renal function
- AEs included
- Grade 3/4 neutropenia (63%)
- Grade 3/4 anemia (31%)
- Manageable CRS in 88% of patients, mostly grades 1–2
The authors noted that earlier intervention before advanced cardiac disease may optimize outcomes, but HBI0101 shows potential to improve organ function and survival in this difficult-to-treat population.
Addressing Cardiac Dysfunction
Further development of therapies targeting deposited amyloid fibrils may be needed to address cardiac dysfunction in AL amyloidosis, according to one report. A single-site retrospective study of 43 patients highlighted the fact that although current systemic AL amyloidosis treatments, including daratumumab- and bortezomib-based regimens, effectively reduce toxic amyloid light chain production, they show limited impact on improving cardiac function. The study showed that 35% of patients experienced clinically significant improvement in cardiac dysfunction, as measured by global longitudinal strain (GLS), but only 10% transitioned from reduced to normal GLS. Most patients (79%) had reduced GLS at baseline, with limited meaningful changes observed across follow-up periods.
—
Jointly provided by the MMRF and RedMedEd.
Support for this activity has been provided through sponsorships from Alexion Pharmaceuticals, Inc.; Pfizer Inc.; and Sanofi US and by an educational grant from Janssen Biotech, Inc., administered by Janssen Scientific Affairs, LLC.
SOUTH SAN FRANCISCO, Calif, February 12, 2025–Opna Bio, a clinical-stage biopharmaceutical company focused on the discovery and development of novel oncology therapeutics, announced today that the FDA has granted orphan drug designation (ODD) to one of its lead programs, OPN-6602, for the treatment of multiple myeloma (MM). OPN-6602 is an oral, small molecule inhibitor of the E1A binding protein (EP300) and CREB-binding protein (CBP) currently being tested in a Phase 1 trial in patients with relapsed or refractory MM.
Read the full press release here.

