
Gary Egan shared 41 years of love and partnership with his late husband, Daniel Holterman, PhD. Together, they built a life filled with laughter, shared passions, and a commitment to making a difference in the world. Gary watched Dan face a 33-year battle with cancer with grace and determination, and Gary continues to honor Dan’s legacy through advocacy and philanthropy.
Dan dedicated his career to cancer research and was deeply involved in the multiple myeloma community as a founding board member and scientific advisor for the Arizona Myeloma Network. “He helped convene a high-level medical research conference annually for ten years,” Gary recalls. “The best specialists in the country leading the fight to cure multiple myeloma were invited.”
Inspired by Dan’s work and resilience, Gary has been supporting the Multiple Myeloma Research Foundation® (MMRF®) by participating in the MMRF Scottsdale Walk/Run for the past two years, rallying friends and family to raise thousands of dollars in Dan’s memory. “The MMRF’s focus on research aligns perfectly with Daniel’s life’s work and reflects our desired philanthropic legacy,” Gary says.
Gary hopes his support of the MMRF will help contribute to the next generation of researchers through the MMRF Research Fellowship Program, giving them the chance to make contributions that will help others — just as Dan did throughout his career.
Gary sees participating in the MMRF Walk/Run as a way to honor Dan while helping families facing multiple myeloma today, and he is honored to be recognized as Scottsdale’s Spirit of Hope Honoree. Through his advocacy, fundraising, and planned giving, Gary continues to carry forward the values he shared with Dan: compassion, perseverance, and a determination to make a difference.

When Carlos Castro was diagnosed with multiple myeloma, he quickly realized the remarkable progress that has been made in treatments — progress and ongoing advancements that allows patients to manage the disease and live longer lives. Inspired by this, he found purpose in supporting research, raising awareness, and participating in clinical trials to contribute to the collective fight against multiple myeloma, which naturally led him to the Multiple Myeloma Research Foundation® (MMRF®), the leading nonprofit organization accelerating a cure for this disease.
The MMRF Walk/Run has become a meaningful way for Carlos to give back. It allows him to support research and innovation while expressing gratitude for the care he has received from his medical team. For Carlos, the event is also a symbol of resilience, representing the challenges he has overcome and the profound life changes experienced by his wife and sons.
Carlos is honored to be recognized as the Los Angeles Spirit of Hope Honoree. “This recognition belongs not only to me, but also to my loving family and friends,” he says. “Their unwavering support, thoughts, prayers, and genuine acts of kindness have uplifted me throughout my journey, making this achievement a shared celebration of resilience and unity.”

Faith and family have been his greatest sources of perseverance. His determination is rooted in his love for his wife and sons, and strengthened by his role as a soccer coach for SCV Patedores and Valencia High School. He reminds his players of the importance of facing adversity, the power of teamwork, and the belief that “victory — no matter how improbable — can be achieved when we stand together.”
Carlos lives by a mantra that guides him through difficult times: “You are not a victim. You may have been challenged, hurt, betrayed, beaten, and discouraged, but nothing has defeated you. Don’t let this experience weaken you — let it build you.”

He is deeply grateful for the many individuals who stand by him — his family, friends, medical team at City of Hope, and the players he coaches. Their encouragement and belief in the mission of the MMRF have made every challenge more bearable and every victory more meaningful.
When Amy McKnett’s mother, Deb, was diagnosed with multiple myeloma, it came as a shock to Amy and her family. Despite Deb’s faith, strength and determination, the disease progressed quickly, and sadly, Deb’s battle with this disease was hard-fought but short. After Deb’s passing in October of 2022, Amy and her family learned of the Multiple Myeloma Research Foundation® (MMRF®) and felt compelled to do something meaningful in her honor.

“Watching her face such a relentless illness with grace and courage changed our lives forever,” Amy shared. The MMRF’s commitment to innovation and patient support resonated with Amy and her family, and they quickly knew they had found a way to channel their grief into purpose.
To celebrate Deb’s life and spirit, Amy decided to create a team for the MMRF Walk/Run Philadelphia, naming her team Nana’s Legacy, to honor her mother’s memory and stand alongside others who have been affected by multiple myeloma. “Every step we take with Nana’s Legacy is a reminder that her spirit lives on — and that together, we’re making a difference.” Amy and her team walk in tribute to the strength, love and resilience that Deb showed not only during her battle with multiple myeloma, but also in life.

Amy is incredibly honored to be recognized as Philadelphia’s Spirit of Hope Honoree this year. For Amy, it’s not just an honor — it’s a reflection of the love, strength and generosity that her mother embodied throughout her life and during her battle with multiple myeloma. “To have our team recognized as the Spirit of Hope is a tribute to her spirit and the legacy she left behind.”
This honor reminds Amy and her family that hope is something we carry forward — not just for ourselves, but for every patient, every family, and every future breakthrough. “It affirms that our efforts matter, and that through love, community, and commitment, we can help bring real change. We accept this honor in her name, and with hearts full of gratitude.”

Through her support of MMRF and by participating in the MMRF Walk/Run Philadelphia event, Amy carries on her mother’s legacy. Her mother persevered through her unwavering faith, boundless love and quiet strength. Her devotion as a caring mother, grandmother, and friend throughout her fight allowed her to keep going during her battle. Deb never let the disease define her — her family gave her purpose, and through getting involved with the MMRF, Amy and Nana’s Legacy hold Deb’s teachings, perseverance, and memory closely.

For years, Gabriela Montes has been a source of comfort, strength and hope for countless patients facing multiple myeloma. Gabriela first heard of the Multiple Myeloma Research Foundation® (MMRF®) through her patients and providers, from where she works as the FFX Infusion Lead Nurse & Cannabis Educator at Virginia Cancer Specialists.
At first, due to her busy schedule, Gabriela was just able to support the organization by donating. For years, the annual MMRF Walk/Run event has been on her calendar — so she could see the impact of her work and support myeloma patients — but this marks the first year that she will be attending and supporting in person. This moment is quite special for Gabriela, and seeing all of the patients walking together in DC will be powerful.
Gabriela is honored to be Washington, DC’s MMRF Spirit of Hope Award honoree this year. She says, “This award represents the deep gratitude from my patients. As nurses, we treat our work with love, kindness, and compassion — and to know that it makes a difference is the greatest honor.”
Apart from her work at Virginia Cancer Specialists, Gabriela finds strength and purpose in many aspects of her life. Her parents taught her that faith, kindness, and compassion are the keys to finding strength and perseverance when faced with adversity. Even in the hardest challenges, Gabriela maintains her unwavering optimism and dedication to bringing hope and healing to those around her.

One of Gabriela’s guiding mantras is “Treat others the way you would like to be treated.” Along with her parents’ teachings, this simple — yet powerful — philosophy shapes the way that Gabriela approaches her work. It reminds her to lead with empathy, patience, and respect.
As Gabriela reflects on her journey, and the honor of receiving the Spirit of Hope Award, she believes that the true Spirit of Hope comes from the patients and survivors of multiple myeloma. Gabriela notes that patients and survivors “continually show me the power of resilience, love, and perseverance.” Gabriela is honored to serve and support these incredible warriors, and looks forward to seeing them on September 27th in Washington, DC.

Nichole Donjè first got involved with the Multiple Myeloma Research Foundation® (MMRF®) after someone presented in her support group. She remembers thinking, “That’s it, I can do this. I wanted to be a part of something. To see if I could make a difference.”
Nichole’s story started with a bad cough, continuous daily fevers, and debilitating chills for four months. She had extreme exhaustion, deep bone pain, and severe brain fog. Nichole realized when it took her half an hour to schedule a meeting, she had to take off work and figure it out. Six specialists and a bone biopsy later, Nichole was diagnosed with multiple myeloma.
For Nichole, starting treatment, as hard as it was, was a relief from what life had been like. Nichole says, “I could think again and walk around the block without being out of breath.”
Nichole quickly found the MMRF as a beacon of hope, information, and resources. As someone who is directly benefitting from the research that’s been done, Nichole decided it was time to give back. “Research is costly and the only way to ensure more treatments are found is to ensure that the funds are there. I know that someday again I will need something new. If I can be a part of making that happen for me and/or for others like me, that’s huge.”

Nichole and her team, The Bloodhounds, have been participating in the MMRF New York City Walk/Run for the past three years. Their team has made an immediate impact, raising over $40,000 in the first two years of participating. Nichole wouldn’t be where she is today without the support of her friends and family. She can always count on her husband Scott, who is also her best friend. “He has been there for me every step of the way. There hasn’t been a treatment or doctor’s visit he hasn’t been to in over three years.” Their dogs, Mr. Molesly and Lily-Belle Clabourne, gave her further purpose. “I have to take care of them, it means I have to keep going.”

Nichole’s incredible community of friends and family, so many of whom either walked or supported the MMRF Walk/Run over the past two years, keep her going as well.
On receiving this year’s Spirit of Hope award for the MMRF New York City Walk/Run, Nichole says that, “It’s a bit hard to put into words.” Nichole remarks that she always hopes that what she does and how she lives will support and help others know they are not alone, that we are all connected in some way. “That someone who saw this energy in me is humbling but also validating that I am living the life I believe I am meant to.”
Throughout her journey, Nichole has found others that are going through similar experiences. Through support groups, or just being connected to someone with myeloma, she found a caring, supportive community. “I have learned that there is something so comforting in being able to speak or simply be present with others who understand, and to be able to ask questions that might be uncomfortable anywhere else.” She found that by helping others, she also helped herself. “We support each other. I’m so grateful for that.”
Nichole can’t wait for this year’s MMRF New York City Walk/Run, saying that the event has given her a sense of strength and purpose. “It’s exciting to have the people in my life come together to make something so important happen. Everyone is here for ourselves and for each other.” Nichole is thankful for the multiple myeloma community and incredibly honored and blown away by being this year’s Spirit of Hope recipient. Nichole is excited to see her team, The Bloodhounds, and the entire NYC MMRF community, on October 11th for yet another impactful event.

Mary Colson-Burns, a lifelong microbiologist and avid runner, was diagnosed with high-risk multiple myeloma in 2018. Through treatment and recovery, she found strength in staying active and supporting the MMRF’s mission. Now the 2025 Spirit of Hope Award recipient, Mary continues to inspire others with her mantra: Keep Moving.
Hear directly from Mary on her story and journey with the MMRF:
In early 2018 I was an avid runner and I had worked as a Clinical Laboratory Scientist since 1971. I had a long history of back pain and was experiencing a flare-up. My physician ordered physical therapy but decided it had been a while since I had an MRI so she ordered one. Later that day she called me at work to tell me I had a significant tumor in my sacrum and it was likely due to multiple myeloma. Having worked in microbiology my entire career, I had heard of myeloma but didn’t know much about it. By the end of the day I had an oncologist, a radiation oncologist, a bone marrow biopsy scheduled, and many tubes of blood drawn.
That year was a whirlwind of radiation, a port insertion, months of chemotherapy, several bone marrow biopsies, cardiac and pulmonary evaluations, and countless lab tests, all in preparation of a stem cell transplant in late 2018, and all while I continued to work full time at the laboratory. It was in 2019, after I returned to work following the transplant, a colleague, who also had multiple myeloma, told me about MMRF and a program called CureCloud where blood was taken to be genetically tested and used for research. I went to the MMRF website and read their Mission, Vision, and Value statements. The core values and the commitment to diversity and equity resonated with me and I knew that this was an organization I could support. The fact that their work was focused on a single disease was also important to me. MMRF has an impressive track record of success in raising money for research, being involved in over 100 clinical trials, bringing new drugs to market, and extending the life expectancies for myeloma patients by 3 times. It is a patient-focused organization, offering valuable resources not only to myeloma patients but to caregivers and those who support myeloma patients. I have participated in many of the webinars and educational sessions and have found them to be extremely valuable.
I first became aware of the MMRF Walk/Run in 2022. Prior to the tumor on my sacrum that led to my diagnosis of multiple myeloma, I was an avid road runner, doing 5Ks, 10Ks, half and full marathons on most weekends. Most of them were fundraisers and I enjoyed that I was raising money while doing something that I loved. I was excited to once again participate. There is something about a group of people walking or running together for a common cause that is uplifting. In 2023, I created a team and got friends and family to join me. We made a morning of it. Last year I brought the team a picnic breakfast for after the walk. People visited and got to know each other. It was a highlight for me and buoyed my spirits.
I am honored to have been chosen for the Spirit of Hope award this year. It is a complete honor to be involved with an organization like MMRF. Being recognized for doing something that I love (physical activity), for something I intensely believe in (medical research), and with the chance that I might inspire others to join me, and to, just maybe, pass on hope to someone with multiple myeloma or their loved ones, what could be better!
Volunteering and fundraising has been a big part of my life. Along with many volunteering opportunities I have had, I became more heavily involved in fundraising when I joined the board of a sliding-fee-scale health clinic and helped develop new fundraising strategies. I have always felt that serving a community is a part of being a good citizen of this world. Having been a clinical microbiologist for 45 years, I know first-hand the importance and value of medical research. I knew that raising funds for an organization with the credentials and reputation of MMRF would be rewarding, regardless of having multiple myeloma.
I have always been an optimistic person but I never really thought about what gives me hope. There are a couple of things that I can put my finger on.
- Science gives me hope for the future. It is the clinical and research scientists who dedicate their work to making life better for people and animals with disease that give me hope. I have seen the benefits of their work, not only in my work but in my multiple myeloma reality.
- Organizations like MMRF and Open Arms of MN that serve people with devastating disease, and are committed to the principles of diversity, inclusion, and equity give me hope.
- The people in my own little world, my family, my friends, and support team, give me hope.
- Lastly, the upcoming generation gives me hope. I see young women who embrace STEM and seek education and careers in the technical fields. I see a new generation that embraces novel ways of doing things and challenges the boundaries of thought. That gives me hope.
But along with hope, I have found that I need strength. In 2010 my husband was unexpectedly killed in an accident. I was devastated. It had just been the two of us for 39 years. I was alone and in shock. I need to find strength to live without him. At his memorial service I ended my tribute to his life by asking the couple of hundred people in the room to consider donating in his honor, to the organization where he volunteered. We raised a significant sum of money. It was gratifying and gave me strength to take his volunteer shift and dedicate my time to that organization. They welcomed me with “Open Arms” and compassion. I felt a connection to him just walking in the building.
Eight years later I was diagnosed with high-risk multiple myeloma and had a grim prognosis. I again needed to find my strength. I found it this time through my love of physical activity. I dedicated myself to keeping fit and healthy. It became a mandatory part of my healthcare. I knew that if I could live through losing my husband, I could do this. I stayed optimistic and looked at each day as a gift. I walk, exercise, and lift weights every day.

I have a favorite mantra that has been with me for a long time, Keep Moving (the name of my team). Every time I am feeling low or not my best I just keep telling myself to keep moving. I tell myself that if I can just keep moving, nothing can catch up with me. It is a mantra that has served me well.
Between my health and fitness regimen and my two wonderful kitties, I am living a fulfilling and healthy life.
Mary Colson-Burns

Jennifer first heard of the Multiple Myeloma Research Foundation® (MMRF®) in the early stages of her husband Donny’s diagnosis, when she was in desperate need to learn as much as possible about this disease. She found a vast amount of knowledge through MMRF’s website and the resources within it. During his journey with multiple myeloma, they had been exposed to many treatments, options, and trials. Jennifer remembers feeling overwhelmed and so very thankful for those who took the time for her, as well as those who went before them to create more support, research, development, and a place for answers. She wanted to be a part of an organization that was making a difference and giving hope when it felt like there was so little.

Although her husband Donny’s battle ended quickly, Jennifer believed that what they lived through was for a purpose greater than the situation they had endured. Jennifer was confident that her MMRF Walk/Run team, TEAMDONNY, was going to “turn this into something good.” Jennifer says, “I wanted desperately to help other individuals and families who were just as confused and overwhelmed as I was. Donny’s journey was not going to be in vain.”
After attending her first MMRF Walk/Run event, Jennifer suddenly felt no longer alone. She recalls looking around and feeling overwhelmed with gratitude, thankful for those walking, living, and fighting. Jennifer remembers, “It took my breath away — I wanted more. To do more and to help more.” For Jennifer, it wasn’t about just raising money — it was support, community building, and walking with others in their journey.
Jennifer and her MMRF Walk/Run team, TEAMDONNY, are committed to the MMRF so that families will have better resources and so that there are more opportunities for advancement in research.

Jennifer is honored to be recognized as Boston’s Spirit of Hope Honoree. She says, “I am speechless. It is truly an honor that we can give HOPE and share our stories to spread awareness of the need for a cure. It’s only because of you all here, for you gave us hope! It’s now our turn to believe and fight for you.”
“This is just the beginning for me. I’m grateful for MMRF and everyone who continues to fight, fundraise and advocate for new treatments. The work being done gives us all more time, more hope, and reasons to keep going.”

- $7M invested in early-stage therapies through the Myeloma Investment Fund®, including in vivo CAR T-cell treatments
- Horizon Clinical Trials testing advanced therapies for relapsed and high-risk patients
- And so much more…

Pam’s story is one of unwavering strength, deep compassion, and a commitment to something far greater than herself. Since being diagnosed with high-risk multiple myeloma in 2021, Pam has faced every challenge — Car-T therapy, a stem cell transplant, chemotherapy, and radiation — with incredible grace and grit. Her journey has become a mission: to build community, raise awareness, and help fund the future of myeloma research.
Honored with the MMRF Torrence Campbell Spirit of Hope Award, Pam represents not only her own perseverance but also the unshakable support of her medical team, devoted friends, and the unwavering love and support of her husband and children.
Since her diagnosis, Pam has tracked her journey each Monday at Sunrise — rain, shine, snow, and storms. Pam wants to make it to 1,080 sunrises to get to 80 years old. She is currently on Sunrise Monday, week #199. Follow her instagram @tinytreks.explorers or FB Tinytreks.

Pam carries a simple but powerful mantra: “Every day is a gift to unwrap.” Each day is cherished, and every effort is made to move research forward. With many treatments already exhausted, the urgency for innovation and progress in new treatments is very real.
Pam’s story is one of resilience, hope, and the belief that together, we can get closer to a cure — even for those with high-risk myeloma.

Carvykti (ciltacabtagene autoleucel) was recently approved for use in myeloma patients who have had at least one prior treatment that didn’t work or stopped working and who no longer respond to Revlimid. Previously, Carvykti was only an option for myeloma patients who had received at least four prior treatments.
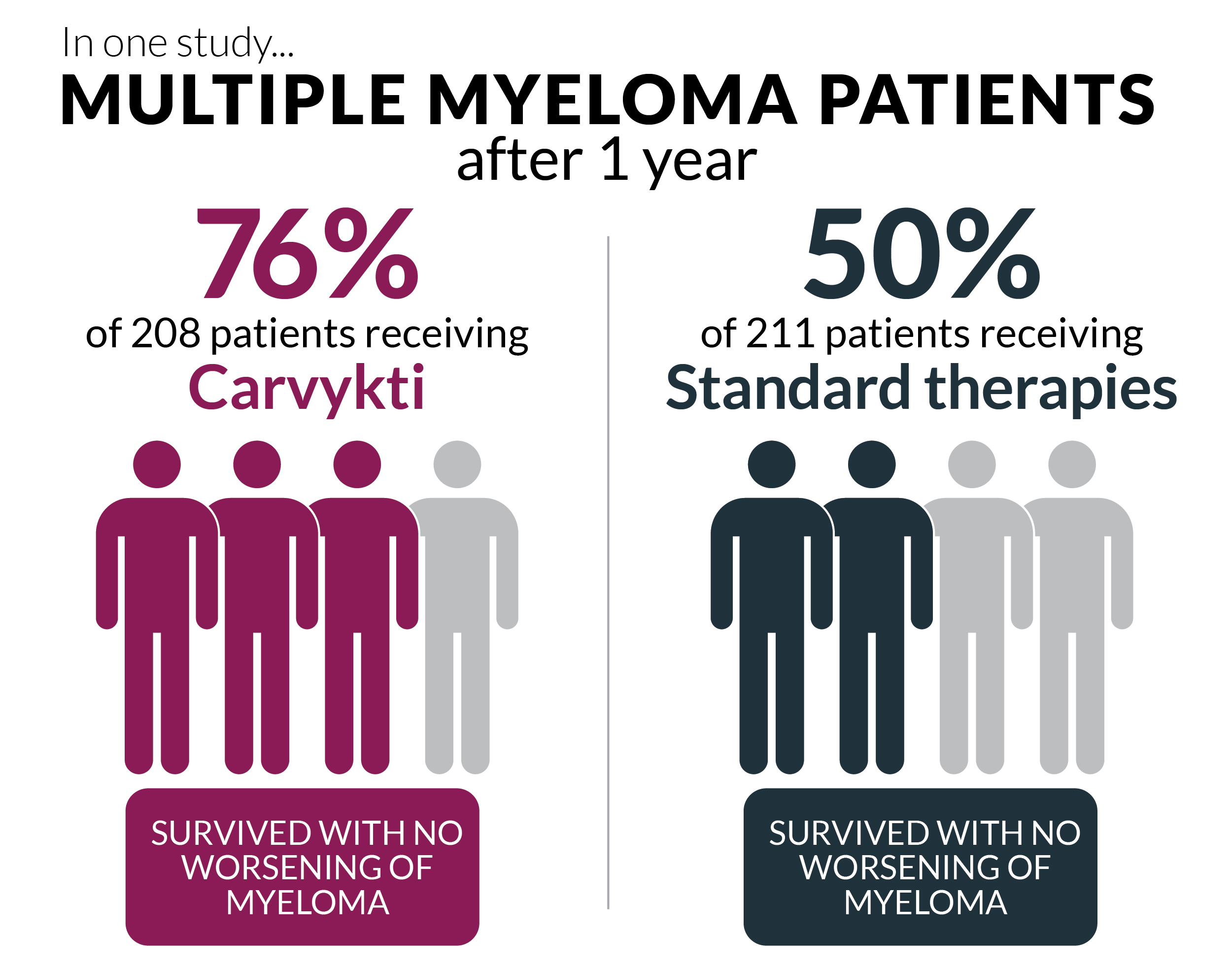
Carvykti is a type of treatment that uses the T cells of your immune system to fight cancer.
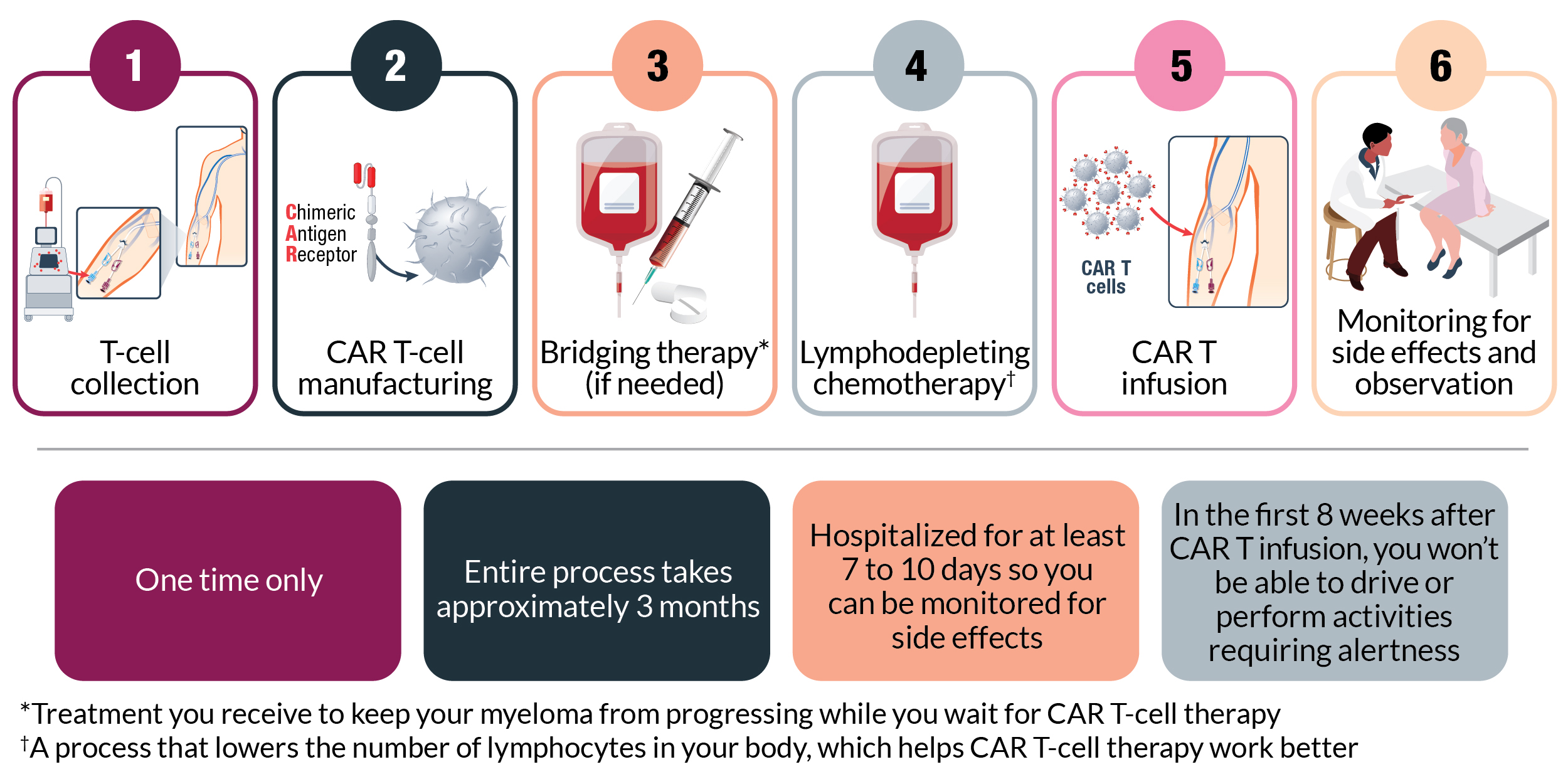
As with other powerful cancer therapies, Carvykti comes with some risks. The most serious potential side effects are cytokine release syndrome and neurologic symptoms, which can be managed by careful monitoring, supportive care, and medication if needed.
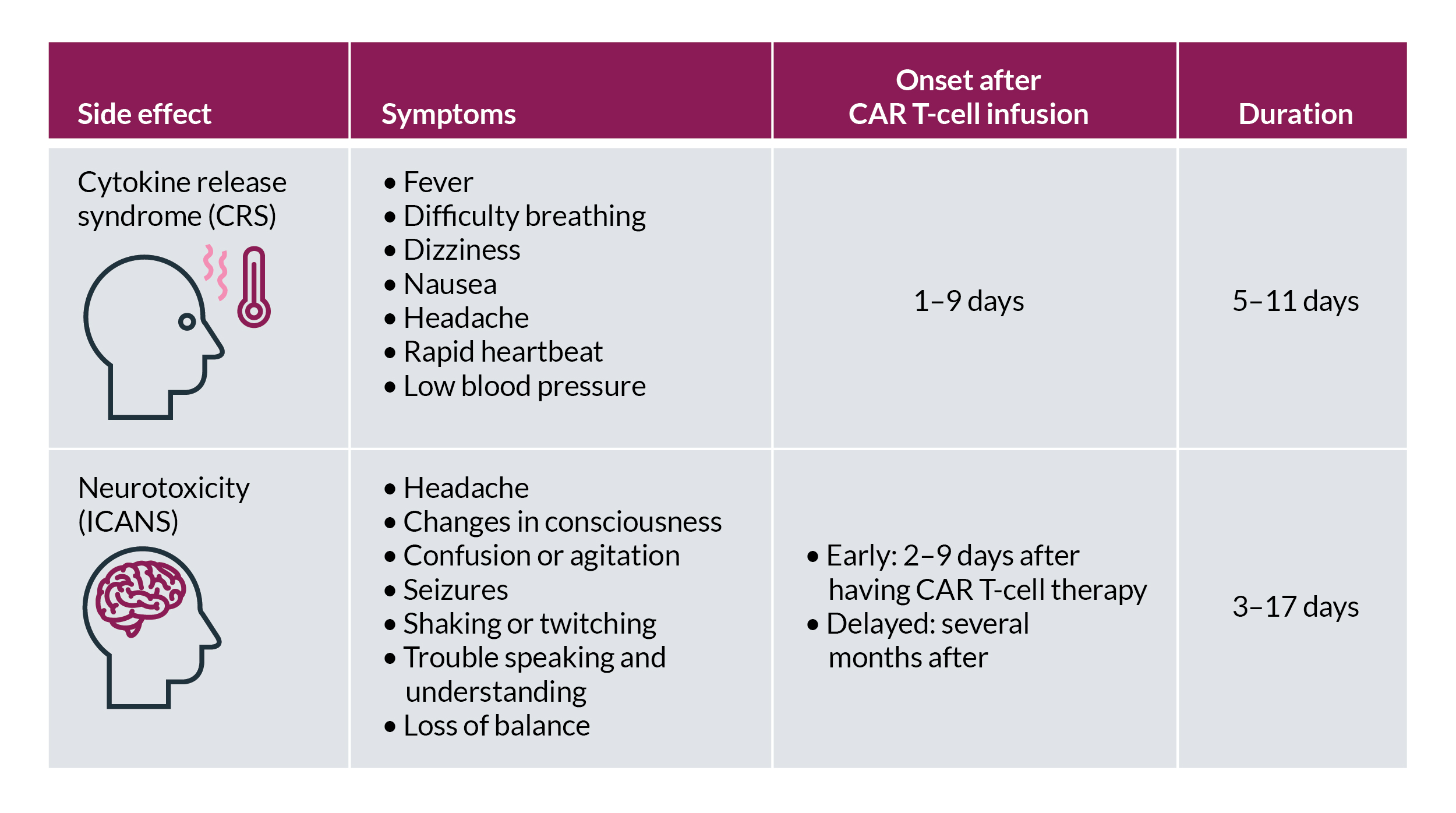
Other common side effects include low blood counts, fatigue, infections, nausea, and muscle or joint pain. You’ll be monitored regularly for side effects, and your care team will provide support to manage them if they develop. Some side effects, particularly neurologic symptoms, can occur well after you receive Carvykti. For this reason, your care team will continue to monitor you for months. Your caregiver should also monitor side effects and communicate any changes to your care team.
Although Carvykti is not for all myeloma patients, it is an exciting step forward for the treatment of relapsed or refractory myeloma. With this updated indication, Carvykti may be available even earlier in the myeloma treatment journey. If you or a loved one has myeloma, talk to your doctor to see if Carvykti might be a good treatment option.
Interested in learning more about Carvykti? Talk to an MMRF patient navigator at the Patient Navigation Center at 1.888.841.MMRF (Monday through Friday, 9:00 am to 7:00 pm ET) or email [email protected].
Additional Resources
Johnson & Johnson. CARVYKTI (ciltacabtagene autoleucel) Patient Support Program.
MMRF. Fast Facts in Myeloma. A Guide to CAR T-Cell Therapy in Myeloma.
MMRF. MMRF Patient Toolkit. Immunotherapy.
MMRF. MMRF Patient Toolkit. Multiple Myeloma Treatment Overview.


