News & Events
Highlights From the 67th ASH Annual Meeting
Multiple myeloma (MM) management is evolving toward highly active, immune‑based, and targeted regimens that aim to deepen and prolong responses from first-line through relapsed/refractory (RRMM) settings.
Several presentations at the 2025 American Society of Hematology Annual Meeting in Orlando introduced new and updated study data on the management of MM, along with potentially significant advancements in therapy. Topics included strategies for deepening treatment responses in pts with newly diagnosed MM (NDMM), emerging roles of cereblon E3 ligase modulators (CELMoDs) and bispecific antibody (bsAb) combinations in early RRMM, and the evolving roles of CAR T and trispecifics.
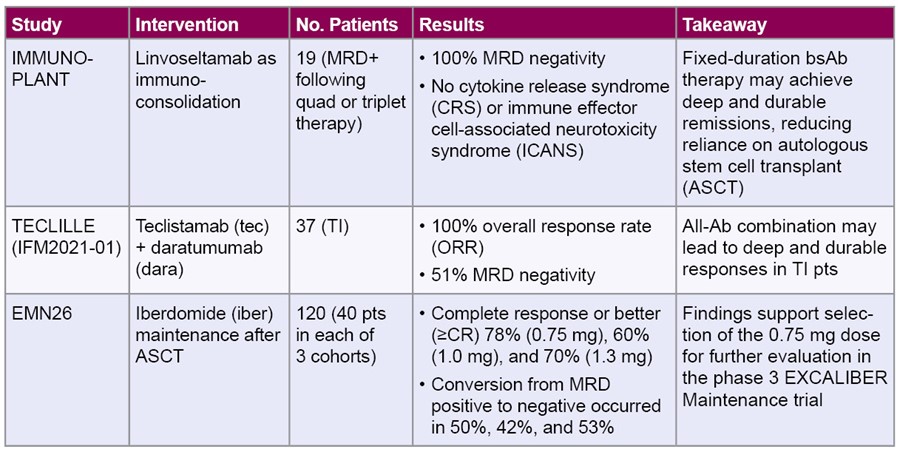
Exploring Strategies to Deepen Responses for NDMM
Across multiple phase 2 and 3 studies, bsAbs and fixed-duration immunoconsolidation strategies show promise in deepening responses—including minimal residual disease (MRD) negativity—when introduced earlier in the treatment course, even in older or transplant-ineligible (TI) pts. Four presentations examined investigational strategies for intensifying therapeutic responses.
Characterizing infection risk in the phase 3 MagnetisMM-6 trial of elra + dara and len in TI or -deferred NDMM pts
One key consideration of bsAb therapy is infections.
The ongoing MagnetisMM-6 trial is evaluating whether adding the BCMA-targeted bsAb elranatamab (elra) to dara and lenalidomide (len; EDR) can improve outcomes compared with the combination of dara, len, and dexamethasone (dex; DRd) in TI or transplant-deferred NDMM pts—a regimen more commonly used outside the U.S. The recommended phase 3 dose was selected from Part 1 of the study based on the early and promising efficacy and manageable safety profile of EDR in pts with RRMM and TI NDMM and was recently presented.
An additional analysis from Part 1 described the characteristics of infections observed in pts who received EDR. Pts (N=34) received EDR, and infections were reported in 70.3% (G3, 18.9%; no G4). One pt had a G5 AE of Candida pneumonia. The most frequent infections were upper respiratory tract infection (21.6%; G3 0%), pneumonias (16.2%; G3 8.1%), and urinary tract infection (10.8%; G3 2.7%). Most first infections (56.8%) occurred within 8 weeks of starting treatment and tended to decrease over time. Anti-infectious prophylaxis was given to 83.8% (Pneumocystis jirovecii pneumonia), 81.1% (viral), 16.2% (fungal), and 10.8% (bacterial) of pts. The majority (91.9%) received Ig replacement. Neutropenia (G3/4) was reported in 73.0% of pts, 8.1% had febrile neutropenia, and most (73%) received granulocyte colony-stimulating factor. Ultimately, treatment with EDR requires close monitoring and prompt intervention to minimize the risk of infection.
Findings from Part 1 supported selection of EDR for phase 3 evaluation based on early and promising efficacy and a manageable safety profile. For Part 2 of this study, approximately 870 adults will be randomized 1:1 to determine whether EDR can improve depth and durability of disease control compared with DRd, with dual primary end points of MRD negativity at 12 months and progression-free survival (PFS).
Notable Outcomes in Early RRMM
Late-breaking phase 3 data (now published) suggest that combining the BCMA-directed bsAb tec with dara may achieve deeper response depths and durability than previously reported in randomized RRMM trials, highlighting the potential clinical relevance of this combination. Although tec is currently approved for use in heavily pretreated RRMM, its evaluation in earlier LOT remains investigational. However, this study highlights the therapeutic potential of more readily accessible, off-the-shelf immunotherapy–based regimen for patients with early RRMM.
Phase 3 MajesTEC-3 study: tec-dara vs dara + dex + either pom or bortezomib (DPd/DVd) in RRMM
- Intervention: Tec-Dara compared with SoC combinations of dara + pom + dex (DPd) or dara + bortezomib + dex (DVd). Tec was administered per a step-up schedule (1.5 mg/kg weekly for cycles 1–2 and 3 mg/kg every 2 weeks for cycles 3–6 and every 4 weeks from cycle 7 onward) with dara at the approved schedule; steroids were discontinued after cycle 1 (day 8)
- Pt population: 587 RRMM pts (Tec-Dara n=291; DPd/DVd n=296) who had 1–3 prior LOT, including a PI and len (len-refractory allowed). Key exclusions included prior BCMA-directed therapy or refractoriness to anti-CD38 antibodies (prior exposure to anti-CD38 was permitted). Median age was 64
- Efficacy: Compared with DPd/DVd, Tec-Dara significantly prolonged PFS (median PFS not reached vs 18.1 months; hazard ratio [HR] 0.17; 95% confidence interval [CI] 0.12–0.23; P<0.0001). The PFS benefit was observed in all pt subgroups, including age ≥75, len-refractory, high-risk cytogenetics, ≥60% bone marrow plasma cells, soft-tissue plasmacytomas, and anti-CD38 exposed
- 36-month PFS: 83.4% (Tec-Dara) vs 29.7% (DPd/DVd)
- Response rates: ≥CR, 81.8% (Tec-Dara) vs 32.1%; ORR, 89.0% vs 75.3%; MRD negativity (10-5 by next-generation sequencing), 58.4% vs 17.1% (all P<0.0001)
- OS significantly favored Tec-Dara (HR 0.46; 95% CI, 0.32–0.65; P<0.0001), including across all subgroups. The 36-month OS rates were 83.3% and 65.0%, and over 90% of Tec-Dara pts alive at 6 months remained alive at 30 months
- Safety: Treatment-emergent adverse events (TEAEs) were as follows (Tec-Dara vs DPd/DVd): grade 3/4 95.1% vs 96.6%; grade 5 7.8% vs 6.2%; serious 70.7% vs 62.4%; discontinuations due to AEs 4.6% vs 5.5%
- Infections (Tec-Dara vs DPd/DVd): any grade 96.5% vs 84.1%; grade 3/4 54.1% vs 43.4%
- CRS: 60.1% (grade 1/2 44.2%/15.9%) with Tec-Dara, with ICANS at 1.1%
- Takeaway: Tec‑Dara demonstrated unprecedented efficacy in early RRMM (ie, after 1-3 LOT), with deep and durable responses and a >80% 3-year PFS and OS, doubling treatment duration compared with DPd/DVd regimens. Safety was manageable, dominated by early-cycle CRS and infections
Predicting Responses in CAR T-Cell Therapy
Paired subanalyses from the CARTITUDE-4 study evaluated risk-specific outcomes in patients treated with ciltacabtagene autoleucel (cilta-cel) to better understand predictors of early relapse and long-term disease control. These analyses reinforce the role of cilta-cel as a highly effective option in early relapsed MM while also identifying patient- and disease-related factors that may influence durability of benefit.
Two presentations focused on distinct risk-based subgroups of cilta-cel-treated patients:
- High-risk subanalysis: CARTITUDE-4 enrolled pts with len-refractory MM after 1 to 3 prior LOT and demonstrated a significant benefit of cilta-cel over established triplets. In a 598-pt
multicenter cohort treated with cilta-cel, ORR and PFS were consistently high but markedly reduced in pts with functional high-risk (FHR) disease, adapted International Myeloma Working Group (IMWG) high-risk cytogenetics, or extramedullary disease (EMD). FHR and adapted IMWG risk independently predicted early relapse (within 18 months), whereas EMD showed a less consistent effect. These findings highlight an identifiable subset susceptible to early disease progression despite cilta-cel treatment - Standard-risk subanalysis: A separate abstract focused on standard-risk cytogenetic pts in CARTITUDE-4. Cilta-cel achieved a 30-month PFS of 80.5% in the as-treated population (n=176 excluding 32 pts who had progressed or died on bridging therapy)—substantially exceeding historical outcomes from later-line trials such as CARTITUDE-1 (59.9%). Pts achieving MRD-negative CR at 12 months had 100% PFS at 30 months, affirming depth of remission as a strong correlate of durable benefit
- Takeaways: Although cilta-cel yields deep and durable responses overall, early relapse risk clusters in pts with FHR or complex cytogenetic risk per adapted IMWG criteria. These findings highlight the importance of recognizing patients with higher-risk features who may benefit from closer monitoring or additional post–CAR T strategies, while reaffirming the strong and sustained benefit observed in standard-risk populations.
Evolving Treatment Approaches in High-Risk Pts
Novel combinations, targeted approaches for molecular subsets, and dual-antigen strategies highlight progress for pts with high-risk features (cytogenetics, extramedullary disease), or limited durability with standard regimen, offering more opportunities to overcome historically poor outcomes.
Talquetamab + tec in pts with RRMM
The phase 1b RedirecTT-1 trial (here and here) investigated the combination of talquetamab (tal, anti-GPRC5D × CD3) and tec (anti-BCMA × CD3) in pts with triple-class-exposed RRMM, including those with true EMD (defined as ≥1 nonradiated plasmacytoma ≥2 cm not contiguous with bone). At a median follow-up of 36.2 months in the recommended phase 2 regimen (tal 0.8 mg/kg + Tec 3.0 mg/kg Q2W), ORR was 79.5% with ≥CR in 61.4% of pts. ORR was 61.1% and 92.3% in pts with or without EMD, respectively. Lower EMD tumor volume correlated with higher ORR (<25 cm2: 90.7%; 25–50 cm2 66.7%; 50 cm2: 65.4%), suggesting prognostic value.
Most common AEs were CRS (80.9% [G3, 2.1%; G4/5, 0%]), neutropenia (74.5% [G3/4, 70.2%]), taste changes (66.0% [all G1/2]), and non-rash skin AEs (62.8% [G3, 2.1%]). Infections occurred in 93.6% of pts (G3/4, 68.1%); the most common was COVID-19 (40.4% [G3/4, 17.0%]). This extended 3-year follow-up confirms that tal + tec provides durable, deep, and potentially survival-prolonging responses with a manageable and predictable safety profile in heavily pretreated RRMM.
Tal + tec demonstrated robust and durable responses in pts with triple-class-exposed RRMM and true EMD, a group with historically poor outcomes. In addition, the safety profile is manageable and in line with known monotherapy toxicities.
Elra + iber in RRMM
In the phase 1b MagnetisMM-30 trial, elra + the oral CELMoD iber showed promising antimyeloma activity and a manageable safety profile in 22 heavily pretreated pts: 4 with EMD; 9 with high-risk cytogenetics defined as t(4;14), t(14;16), or del(17p); 1 with Revised International Staging System stage III; and 2 with ≥50% baseline bone marrow plasma cells. The ORR (unconfirmed) was 90.9%, with nearly half (45.5%) achieving ≥CR. Elra + iber demonstrated strong early efficacy and acceptable tolerability in RRMM, supporting continued evaluation.
Next-Generation Therapies: CAR T
Updated registrational data and first-in-human studies showcase the rapid evolution of CAR T-cell therapy, including next-generation constructs, dual-targeting strategies, and gene-therapy approaches designed to simplify manufacturing while extending responses, even in pts with disease refractory to multiple prior LOT. Extending dual-target CAR T innovation beyond MM, early-phase data explore feasibility and activity in relapsed AL amyloidosis.
Phase 2 iMMagine 1 trial of anito-cel for RRMM: update
- Intervention: Anitocabtagene autoleucel (anito-cel) is an autologous anti-BCMA CAR T-cell therapy using a novel D-domain binder designed for high transduction efficiency, stable CAR expression, and reduced tonic signaling
- Pt population: 117 pts (median age: 64 years [range 38–78]) who were refractory to the last LOT; 86% triple-class refractory; 40% penta-drug refractory; 15% with EMD; 38% with high-risk cytogenetics
- Efficacy: ORR was 97%, and the complete/stringent complete response (CR/sCR) was 68%. MRD negativity rate was 93% at 10-5 (70/75 evaluable) and 78% at 10-6 (53/68). Time to first response and MRD negativity averaged (median) 1 month. PFS rates at 12 and 18 months were 79% and 66%, respectively. OS rates at 12 and 18 months were 95% and 90%, respectively
- Safety: Common grade 3/4 AEs included neutropenia 66%, anemia 24%, and thrombocytopenia 24%. Grade 3/4 infections occurred in 9%. CRS was mostly grade 1 (70%), with 1 grade 5 event. ICANS emerged in 8% (mostly grade 1–2; 1 grade 3). No immune effector cell-associated enterocolitis or secondary T-cell malignancies were observed
- Takeaway: Anito-cel demonstrates deep, durable responses and manageable toxicity in a highly refractory RRMM population. The absence of delayed or atypical neurotoxicities supported favorable long-term safety
Dual-targeting BCMA and CD19 FasTCAR-T (GC012F/AZD0120): platform innovation and clinical applications
GC012F/AZD0120 is an autologous dual-target BCMA/CD19 CAR T therapy manufactured via the FasTCAR-T next-day platform (a manufacturing approach designed to substantially shorten development time, potentially reducing the need for prolonged bridging therapy and allowing earlier intervention). In 30 treated NDMM pts (following 2 cycles of RVd induction therapy), the ORR was 100%, with a 97% sCR. All pts achieved MRD negativity (10-6), and just over 80% maintained MRD negativity beyond 12 months. After a median follow-up of 30 months, neither PFS nor OS had been reached, with 30-month PFS and OS rates of 88% and 92%, respectively. GC012F/AZD0120 was well tolerated, with grade 1–2 CRS in 33% of pts and no neurotoxicity. GC012F/AZD0120 demonstrates exceptional efficacy, durable MRD negativity, and a favorable safety profile, warranting larger confirmatory trials.
The FasTCAR-T platform is now being explored beyond MM in relapsed or refractory (RR) light chain (AL) amyloidosis, a plasma cell (PC) disorder and occurs in 10% to 15% of MM pts. In about one third of pts, second-line therapy is needed within 22 months following first-line dara + CyBorD. Yet there are no approved therapies for relapsed or refractory (RR) AL amyloidosis. New therapies inducing deep and durable responses are needed to extend survival.
ALACRITY, a phase 1b/2 trial, will evaluate the use of AZD0120 in RR AL amyloidosis. Pts enrolled on the study must have relapsed or be refractory to ≥1 prior line of anti-PC-directed therapy, including a CD38 monoclonal antibody and a PI. Pts who have had prior CAR-T or BCMA-directed therapy, or any FDA-approved or investigational T cell engaging therapy (eg, bispecifics/trispecifics) at any target within the last 6 months will be excluded. The recommended phase 2 dose (RP2D) will be selected based on safety, preliminary efficacy, cellular kinetics, and pharmacodynamics. Phase 2 will enroll 79 pts at the RP2D.
MRD-negative outcomes following a novel in vivo gene therapy generating BCMA CAR T cells in RRMM
A late-breaking first-in-human phase 1 study evaluated KLN-1010, an investigational in vivo BCMA-directed CAR T–gene therapy designed to generate CAR T cells without ex vivo manufacturing or lymphodepletion, representing a truly next-generation approach to cellular therapy. In this very small cohort of three heavily pretreated, high-risk patients, treatment was feasible and generally well tolerated, with all patients achieving early MRD-negative responses—findings that warrant cautious interpretation but underscore the potential of in vivo CAR T strategies.
Next-Generation Therapies: Bispecifics and Trispecifics
Emerging bispecific and trispecific platforms demonstrate improved convenience, manageable safety, and expanding clinical versatility, including post-CAR T consolidation and novel multitarget engagement that may further extend the reach of T-cell redirection strategies.
Kilimanjaro: a phase 1b dose-escalation and safety expansion study of etentamig + pom + dex in RRMM
Etentamig (a BCMA × CD3 bsAb with unique design features that can reduce CRS, extend half-life, and offer a convenient monthly dosing schedule) was evaluated in combination with pomalidomide (pom) + dex in 85 RRMM pts. Pts had a median of 4 prior LOT; 73% were triple-class refractory; 58% were exposed to pom (half were pom-refractory). ORR in evaluable pts was 81% (72% ≥VGPR). The most common G3/4 hematologic AEs were neutropenia (788%), anemia (28%), and thrombocytopenia (22%). CRS occurred in 37% of pts (G1, 25%; G2, 12%; G≥3, 0%). ICANS was reported in 7% of pts (most G1/2; 1 pt with G3). Infections (G3/4) occurred in 49% (most common, pneumonia). Etentamig regimens will be explored in a randomized phase 3 study based on these results.
Non–BCMA-targeted bsAbs in RRMM
Early-phase studies of non-BCMA T-cell–redirecting therapies highlight the growing role of alternative targets in RRMM. Cevostamab, a CD3 × FcRH5 bsAb, demonstrated manageable safety—primarily low-grade CRS and hematologic toxicities—and meaningful activity both as post–BCMA CAR T consolidation (with high rates of MRD negativity) and as a subcutaneous regimen in heavily pretreated patients, achieving ORRs of approximately 40%. In parallel, the first-in-human trispecific antibody IBI3003 targeting GPRC5D, BCMA, and CD3 showed promising early efficacy with ORRs ranging from 50% to 100% across dose levels, including in patients with prior BCMA or GPRC5D exposure and extramedullary disease, with toxicity that was generally manageable.
Jointly provided by the MMRF and RedMedEd.
Support for this activity has been provided through sponsorships from Alexion Pharmaceuticals, Inc.; Legend Biotech USA Inc.; Pfizer Inc.; and Sanofi and by educational grants from AbbVie Inc. and Janssen Biotech, Inc., administered by Janssen Scientific Affairs, Affairs, LLC.